Characterization of two β-galactosidases LacZ and WspA1 from Nostoc flagelliforme with focus on the latter's central active region
- PMID: 34531460
- PMCID: PMC8445988
- DOI: 10.1038/s41598-021-97929-6
Characterization of two β-galactosidases LacZ and WspA1 from Nostoc flagelliforme with focus on the latter's central active region
Abstract
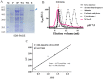
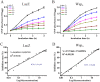
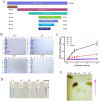
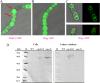
The identification and characterization of new β-galactosidases will provide diverse candidate enzymes for use in food processing industry. In this study, two β-galactosidases, Nf-LacZ and WspA1, from the terrestrial cyanobacterium Nostoc flagelliforme were heterologously expressed in Escherichia coli, followed by purification and biochemical characterization. Nf-LacZ was characterized to have an optimum activity at 40 °C and pH 6.5, different from that (45 °C and pH 8.0) of WspA1. Two enzymes had a similar Michaelis constant (Km = 0.5 mmol/liter) against the substrate o-nitrophenyl-β-D-galactopyranoside. Their activities could be inhibited by galactostatin bisulfite, with IC50 values of 0.59 µM for Nf-LacZ and 1.18 µM for WspA1, respectively. Gel filtration analysis suggested that the active form of WspA1 was a dimer, while Nf-LacZ was functional as a larger multimer. WspA1 was further characterized by the truncation test, and its minimum central region was found to be from residues 188 to 301, having both the glycosyl hydrolytic and transgalactosylation activities. Finally, transgenic analysis with the GFP reporter protein found that the N-terminus of WspA1 (35 aa) might play a special role in the export of WspA1 from cells. In summary, this study characterized two cyanobacterial β-galactosidases for potential applications in food industry.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Unique WSPA protein from terrestrial macroscopic cyanobacteria can confer resistance to osmotic stress in transgenic plants.World J Microbiol Biotechnol. 2014 Sep;30(9):2361-9. doi: 10.1007/s11274-014-1661-9. Epub 2014 May 9. World J Microbiol Biotechnol. 2014. PMID: 24811897
-
A novel cold-active β-D-galactosidase from the Paracoccus sp. 32d--gene cloning, purification and characterization.Microb Cell Fact. 2011 Dec 13;10:108. doi: 10.1186/1475-2859-10-108. Microb Cell Fact. 2011. PMID: 22166118 Free PMC article.
-
Biochemical characterization of a beta-galactosidase with a low temperature optimum obtained from an Antarctic arthrobacter isolate.J Bacteriol. 2003 Sep;185(18):5473-82. doi: 10.1128/JB.185.18.5473-5482.2003. J Bacteriol. 2003. PMID: 12949099 Free PMC article.
-
Characterization of a psychrotrophic Arthrobacter gene and its cold-active beta-galactosidase.Appl Environ Microbiol. 1994 Dec;60(12):4544-52. doi: 10.1128/aem.60.12.4544-4552.1994. Appl Environ Microbiol. 1994. PMID: 7811090 Free PMC article.
-
Screening novel β-galactosidases from a sequence-based metagenome and characterization of an alkaline β-galactosidase for the enzymatic synthesis of galactooligosaccharides.Protein Expr Purif. 2019 Mar;155:104-111. doi: 10.1016/j.pep.2018.12.001. Epub 2018 Dec 4. Protein Expr Purif. 2019. PMID: 30529535
Cited by
-
Molecular Mechanisms of Nostoc flagelliforme Environmental Adaptation: A Comprehensive Review.Plants (Basel). 2025 May 22;14(11):1582. doi: 10.3390/plants14111582. Plants (Basel). 2025. PMID: 40508256 Free PMC article. Review.
-
Structure revision of scytonemin imine and its relationship to scytonemin chromism and cyanobacterial adaptability.Sci Rep. 2025 Jul 15;15(1):25630. doi: 10.1038/s41598-025-10419-x. Sci Rep. 2025. PMID: 40664929 Free PMC article.
-
Biochemical Insights into a Novel Family 2 Glycoside Hydrolase with Both β-1,3-Galactosidase and β-1,4-Galactosidase Activity from the Arctic.Mar Drugs. 2023 Sep 29;21(10):521. doi: 10.3390/md21100521. Mar Drugs. 2023. PMID: 37888456 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

