Therapeutic Plasma Exchange in Myasthenia Gravis: A Systematic Literature Review and Meta-Analysis of Comparative Evidence
- PMID: 34531809
- PMCID: PMC8439193
- DOI: 10.3389/fneur.2021.662856
Therapeutic Plasma Exchange in Myasthenia Gravis: A Systematic Literature Review and Meta-Analysis of Comparative Evidence
Abstract
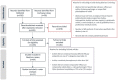
Background: Patients with Myasthenia Gravis (MG) can be treated acutely with therapeutic plasma exchange (TPE) or intravenous immune globulin (IVIG). To date, there is no definitive understanding of which of the two treatments is more effective and safer. The purpose of this study was to systematically review the literature on the comparative efficacy and safety of TPE to other available treatments for MG. Methods: A systematic literature search for studies published between 1997 and 2017 was performed per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using two database sources, MEDLINE (through the PubMed database) and Cochrane Library. Results: The search strategy resulted in 535 articles whose abstracts were reviewed. Among these, 165 full texts articles were reviewed for eligibility and 101 articles were excluded. Of the 165 articles, 64 articles were included for a systematic literature and 11 articles for a meta-analysis. Conclusions: This systematic literature review and meta-analysis of treatment options showed that there was a higher response rate with TPE than IVIG in acute MG patients and patients undergoing thymectomy. There was no difference in mortality between the two treatment options. Our findings highlight the need for additional randomized clinical trials in these patients with MG.
Keywords: autoimmune disorders; myasthenia (myasthenia gravis-MG); neurological diseases; plasmapheresis; therapies and management.
Copyright © 2021 Ipe, Davis and Raval.
Conflict of interest statement
TSI and JSR are consultants for Terumo Blood and Cell Technologies. ARD was an employee of Terumo Blood and Cell Technologies.
References
-
- Abbas Jowkar C, Lorenzo N. Myasthenia gravis: practice essentials, background, anatomy. Medscape. (2017). Available online at: https://emedicine.medscape.com/article/1171206-overview (accessed September, 2019).
Publication types
LinkOut - more resources
Full Text Sources
Medical