Overcoming the Pregnane X Receptor Liability: Rational Design to Eliminate PXR-Mediated CYP Induction
- PMID: 34531948
- PMCID: PMC8436247
- DOI: 10.1021/acsmedchemlett.1c00187
Overcoming the Pregnane X Receptor Liability: Rational Design to Eliminate PXR-Mediated CYP Induction
Abstract
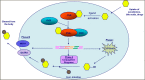
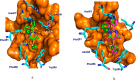
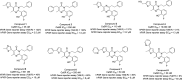
The pregnane X receptor (PXR) regulates expression of proteins responsible for all three phases required for the detoxification mechanism, which include CYP450 enzymes, phase II enzymes, and multidrug efflux pumps. Therefore, PXR is a prominent receptor that is responsible for xenobiotic excretion and drug-drug interactions. Pyrimidinone 1 is an antagonist of the calcium sensing receptor (CaSR) and a strong activator of PXR. Repeat oral administration revealed diminished exposures over time, which prohibited further progression. A medicinal chemistry campaign was initiated to understand and abolish activation of PXR in order to increase systemic exposures. Rational structure-activity relationship investigations utilizing cocrystal structures and a de novo pharmacophore model resulted in compounds devoid of PXR activation. These studies culminated in the first orally active CaSR antagonist 8 suitable for progression. Cocrystallography, the pharmacophore model employed, and additional observations reported herein supported rational elimination of PXR activation and have applicability across diverse chemical classes to help erase PXR-driven drug-drug interactions.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




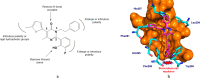
References
-
- Jones S. A.; Moore L. B.; Shenk J. L.; Wisely G. B.; Hamilton G. A.; McKee D. D.; Tomkinson N. C.; LeCluyse E. L.; Lambert M. H.; Willson T. M.; Kliewer S. A.; Moore J. T. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol. Endocrinol. 2000, 14, 27–39. 10.1210/mend.14.1.0409. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

