Development of an amplified luminescent proximity homogeneous assay for the detection of sulfonamides in animal-derived products
- PMID: 34532005
- PMCID: PMC8441374
- DOI: 10.1002/fsn3.2443
Development of an amplified luminescent proximity homogeneous assay for the detection of sulfonamides in animal-derived products
Abstract
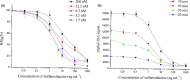
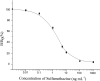
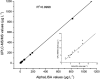
In this study, we carried out an amplified luminescent proximity homogeneous assay (AlphaLISA) to detect sulfonamides (SAs) antibiotic residues in plasma, milk, pork, chicken, and fish. The SAs AlphaLISA method can detect 13 SAs with half-inhibitory concentration (IC50) 2.11-29.77 ng/ml. The detection level of those SAs was 0.3-41.12 ng/ml in matrices, which satisfied the maximum residue limit (MRL) of the European Union, United States, and China. Our recoveries are in the range of 88% to 116.8% with a coefficient of variation less than 9.3% for different spiked food samples. We observed a good correlation between the AlphaLISA and liquid chromatography-tandem mass spectrometry (LC-MS/MS) with blood samples from injected rabbits. The established AlphaLISA method provided a no-washing, rapid, high-throughput screening tool for SAs in food quality control, which is suitable for small-volume samples.
Keywords: amplified luminescent proximity homogeneous assay (AlphaLISA); food quality control; sulfamethazine; sulfonamides.
© 2021 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
1No conflict of interest declared.
Figures


Similar articles
-
Sensitive amplified luminescent proximity homogeneous assay for the quantitative detection of CA242.J Immunol Methods. 2023 Jun;517:113487. doi: 10.1016/j.jim.2023.113487. Epub 2023 May 6. J Immunol Methods. 2023. PMID: 37156407
-
Okadaic Acid Detection through a Rapid and Sensitive Amplified Luminescent Proximity Homogeneous Assay.Toxins (Basel). 2023 Aug 14;15(8):501. doi: 10.3390/toxins15080501. Toxins (Basel). 2023. PMID: 37624258 Free PMC article.
-
Regulating the Growth Rate of Gold Nanobipyramids via a HCl-NADH-Ascorbic Acid System toward a Dual-Channel Multicolor Colorimetric Immunoassay for Simultaneously Screening and Detecting Multiple Sulfonamides.Anal Chem. 2023 Jul 11;95(27):10438-10447. doi: 10.1021/acs.analchem.3c01928. Epub 2023 Jun 29. Anal Chem. 2023. PMID: 37382204
-
[Determination of nine food-borne stimulant drug residues in pork, egg, and milk by ultra-performance liquid chromatography-tandem mass spectrometry].Se Pu. 2022 Feb 8;40(2):148-155. doi: 10.3724/SP.J.1123.2021.04005. Se Pu. 2022. PMID: 35080161 Free PMC article. Chinese.
-
Rapid and sensitive detection of botulinum toxin type A in complex sample matrices by AlphaLISA.Front Public Health. 2022 Oct 20;10:987517. doi: 10.3389/fpubh.2022.987517. eCollection 2022. Front Public Health. 2022. PMID: 36339146 Free PMC article.
Cited by
-
A dual-mode fluorometric/colorimetric sensor for sulfadimethoxine detection based on Prussian blue nanoparticles and carbon dots.Mikrochim Acta. 2024 Apr 23;191(5):284. doi: 10.1007/s00604-024-06358-5. Mikrochim Acta. 2024. PMID: 38652331
References
-
- Adesiyun, A. A., Nkuna, C., Mokgoatlheng‐Mamogobo, M., Malepe, K., & Simanda, L. (2020). Food safety risk posed to consumers of table eggs from layer farms in Gauteng Province, South Africa: Prevalence of Salmonella species and Escherichia coli, antimicrobial residues, and antimicrobial resistant bacteria. Journal of Food Safety, 40, e12783. 10.1111/jfs.12783 - DOI
-
- Administration, F. A. D. (2018). Tolerances for residues of new animal drugs in food; Title 21: Food and drugs, part 556. Code of federal regulations. Office of the Federal Register: Washington, DC, USA.
-
- Agriculture, P.M.O , (2002). Bulletin No 235, maximum residue limits of veterinary medicinal products in foodstuffs and animal origin. Beijing, China.
LinkOut - more resources
Full Text Sources

