Microwave ablation for colorectal cancer metastasis to the liver: a single-center retrospective analysis
- PMID: 34532102
- PMCID: PMC8421889
- DOI: 10.21037/jgo-21-159
Microwave ablation for colorectal cancer metastasis to the liver: a single-center retrospective analysis
Abstract
Background: The purpose of this study is to evaluate the safety and intermediate-term efficacy of percutaneous microwave (MW) ablation for the treatment of colorectal liver metastases (CRLM) at a single institution.
Methods: A retrospective review was performed of all CRLM treated with MW ablation from 3/2011 to 7/2020 (102 tumors; 72 procedures; 57 patients). Mean age was 60 years (range, 36-88) and mean tumor size was 1.8 cm (range, 0.5-5.0 cm). The patient population included 19 patients with extra-hepatic disease. Chemotherapy (pre- and/or post-ablation) was given in 98% of patients. Forty-five sessions were preceded by other focal CRLM treatments including resection, ablation, radiation, and radioembolization. Kaplan-Meier curves were used to estimate local tumor progression-free survival (LTPFS), disease-free survival (DFS), and overall survival (OS) and multivariate analysis (Cox Proportional Hazards model) was used to test predictors of OS.
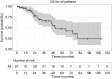
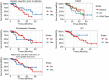
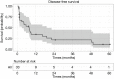
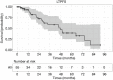
Results: Technical success (complete ablation) was 100% and median follow-up was 42 months (range, 1-112). There was a 4% major complication rate and an overall complication rate of 8%. Local tumor progression (LTP) rate during the entire study period was 4/98 (4%), in which 2 were retreated with MW ablation for a secondary LTP-rate of 2%. LTP-free survival at 1, 3, and 5 years was 93%, 58%, and 39% and median LTP-free survival was 48 months. OS at 1, 3, and 5 years was 96%, 66%, 47% and median OS was 52 months. There were no statistically significant predictors of OS.
Conclusions: MW ablation of hepatic colorectal liver metastases appears safe with excellent local tumor control and prolonged survival compared to historical controls in selected patients. Further comparative studies with other local treatment strategies appear indicated.
Keywords: Colorectal cancer; ablation; hepatic metastasis; liver targeted therapy; treatment of metastasis.
2021 Journal of Gastrointestinal Oncology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-159). TJZ receives research funding from HistoSonics Inc. and Ethicon Inc., is a shareholder of HistoSonics Inc., and is a consultant of Ethicon Inc.. SJL receives research funding from Incyte and Agios. JLH is a shareholder of Elucent Medical, Accure, HistoSonics Inc., and Cellectar and is a consultant of Ethicon Inc.. MGL received research funding previously from Philips and Ethicon Inc.. DA is a consultant of PatientPort. DD is a consultant of Array, Pfizer, Acrotech, MEI Pharma, Taiho, Bristol Myers Squibb, Promega, and Bayer and receives research funding from Merck, Revolution Medicine, Bayer, Promega, Genentech, and EMD Serono. NU is a consultant of QED, Ipsen, Taiho Inc., Incyte, and AstraZeneca, receives research funding from Taiho Inc., Eli Lilly, Ipsen, and EMD Serono, and has long position holdings in Natera and Exact Sciences. SAW is a consultant of Ethicon Inc.. PFL is a consultant of HistoSonics Inc. and Ethicon Inc., is a shareholder of HistoSonics Inc., and receives research funding from HistoSonics Inc.. MA is a consultant of Ethicon Inc.. FTL is a board member of HistoSonics Inc., is a consultant of HistoSonics Inc.. and Ethicon Inc., is a shareholder of HistoSonics Inc., receives research funding from HistoSonics Inc. and Ethicon Inc., and has patents/royalties from Medtronic. The other authors have no conflicts of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources
