Optimal adjuvant chemotherapy completion time for stage III colon cancer: a cohort study
- PMID: 34532110
- PMCID: PMC8421879
- DOI: 10.21037/jgo-21-317
Optimal adjuvant chemotherapy completion time for stage III colon cancer: a cohort study
Abstract
Background: Adjuvant chemotherapy for 6 months following surgery is the standard treatment plan for stage III colon cancer. The aim of the present study was to determine whether the adjuvant chemotherapy completion time for stage III colon cancer had an effect on prognosis and cut-off time that affected the prognosis.
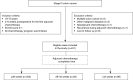
Methods: This was a retrospective study of stage III colon cancer patients who completed adjuvant chemotherapy at Guangzhou Red Cross Hospital from January 2010 to December 2017. Univariate and multivariate analyses were used to determine the association between adjuvant chemotherapy completion time and the 3-year disease-free survival (DFS). The restricted cubic spline model was used to analyze the cut-off time that affected the 3-year DFS.
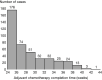
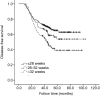
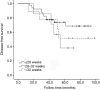
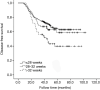
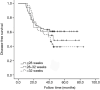
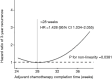
Results: A total of 431 patients were included in the study. The 3-year DFS was associated with a combination of obstruction or perforation, preoperative serum carcino-embryonic antigen (CEA) concentration, T stage, N stage, pathological stage, and adjuvant chemotherapy completion time in the univariate analysis (P<0.05). A combination of obstruction or perforation, preoperative serum CEA concentration, N stage, and adjuvant chemotherapy completion time were independent prognostic factors in the multivariate analysis (P<0.05). The cut-off time was 28 weeks for adjuvant chemotherapy completion time in the restricted cubic spline model analysis. For those whose adjuvant chemotherapy completion time was >28 weeks, the risk of 3-year recurrence was 1.428 times higher compared with those whose adjuvant chemotherapy completion time was ≤28 weeks. [P=0.032, 95% confidence interval (CI): 1.034-2.055].
Conclusions: The 3-year DFS of stage III colon cancer was related to the adjuvant chemotherapy completion time. For those who completed adjuvant chemotherapy >28 weeks, the risk of 3-year recurrence increased.
Keywords: Colon cancer; adjuvant chemotherapy; completion time; disease-free survival (DFS).
2021 Journal of Gastrointestinal Oncology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-317). The authors have no conflicts of interest to declare.
Figures
References
-
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9. - PubMed
LinkOut - more resources
Full Text Sources
