Insights Into the Pathologic Roles and Regulation of Eukaryotic Elongation Factor-2 Kinase
- PMID: 34532346
- PMCID: PMC8438118
- DOI: 10.3389/fmolb.2021.727863
Insights Into the Pathologic Roles and Regulation of Eukaryotic Elongation Factor-2 Kinase
Abstract
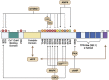
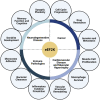
Eukaryotic Elongation Factor-2 Kinase (eEF2K) acts as a negative regulator of protein synthesis, translation, and cell growth. As a structurally unique member of the alpha-kinase family, eEF2K is essential to cell survival under stressful conditions, as it contributes to both cell viability and proliferation. Known as the modulator of the global rate of protein translation, eEF2K inhibits eEF2 (eukaryotic Elongation Factor 2) and decreases translation elongation when active. eEF2K is regulated by various mechanisms, including phosphorylation through residues and autophosphorylation. Specifically, this protein kinase is downregulated through the phosphorylation of multiple sites via mTOR signaling and upregulated via the AMPK pathway. eEF2K plays important roles in numerous biological systems, including neurology, cardiology, myology, and immunology. This review provides further insights into the current roles of eEF2K and its potential to be explored as a therapeutic target for drug development.
Keywords: AMPK; cancer; drug development; eEF2K; immunometabolism; mTORC1; protein kinase; signaling pathways.
Copyright © 2021 Ballard, Peng, Das, Kumar, Wang, Ren, Xiong, Ren, Yang and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abramczyk O., Tavares C. D. J., Devkota A. K., Ryazanov A. G., Turk B. E., Riggs A. F., et al. (2011). Purification and Characterization of Tagless Recombinant Human Elongation Factor 2 Kinase (eEF-2K) Expressed in Escherichia coli . Protein Expr. Purif. 79, 237–244. 10.1016/j.pep.2011.05.005 - DOI - PMC - PubMed
-
- Arora S., Yang J. M., Kinzy T. G., Utsumi R., Okamoto T., Kitayama T., et al. (2003). Identification and Characterization of an Inhibitor of Eukaryotic Elongation Factor 2 Kinase against Human Cancer Cell Lines. Cancer Res. 63, 6894–6899. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

