Animal models of corneal endothelial dysfunction to facilitate development of novel therapies
- PMID: 34532408
- PMCID: PMC8421955
- DOI: 10.21037/atm-20-4389
Animal models of corneal endothelial dysfunction to facilitate development of novel therapies
Abstract
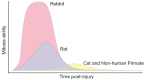
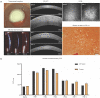
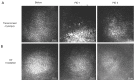
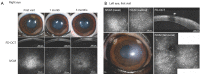
Progressive corneal endothelial disease eventually leads to corneal edema and vision loss due to the limited regenerative capacity of the corneal endothelium in vivo and is a major indication for corneal transplantation. Despite the relatively high success rate of corneal transplantation, there remains a pressing global clinical need to identify improved therapeutic strategies to address this debilitating condition. To evaluate the safety and efficacy of novel therapeutics, there is a growing demand for pre-clinical animal models of corneal endothelial dysfunction. In this review, experimentally induced, spontaneously occurring and genetically modified animal models of corneal endothelial dysfunction are described to assist researchers in making informed decisions regarding the selection of the most appropriate animal models to meet their research goals.
Keywords: Corneal endothelium; corneal endothelial disease; corneal endothelial injury; fuchs endothelial corneal dystrophy; pre-clinical animal models.
2021 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-20-4389). The series “Novel Tools and Therapies for Ocular Regeneration” was commissioned by the editorial office without any funding or sponsorship. CJM reports grants from National Eye Institute, and an unrestricted grant from the family of Claire Burns, during the conduct of the study. SMT reports grants from NEI, and an unrestricted grant from the family of Claire Burns, during the conduct of the study. The authors have no other conflicts of interest to declare.
Figures
Similar articles
-
Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis.Prog Retin Eye Res. 2021 Jan;80:100863. doi: 10.1016/j.preteyeres.2020.100863. Epub 2020 May 8. Prog Retin Eye Res. 2021. PMID: 32438095 Free PMC article. Review.
-
Recent and Evolving Therapies in the Management of Endothelial Diseases.Semin Ophthalmol. 2023 Feb;38(2):207-215. doi: 10.1080/08820538.2022.2152717. Epub 2022 Dec 29. Semin Ophthalmol. 2023. PMID: 36582139 Review.
-
Regenerative capacity of the corneal transition zone for endothelial cell therapy.Stem Cell Res Ther. 2020 Dec 4;11(1):523. doi: 10.1186/s13287-020-02046-2. Stem Cell Res Ther. 2020. PMID: 33276809 Free PMC article. Review.
-
Experimental models of corneal endothelial cell therapy and translational challenges to clinical practice.Exp Eye Res. 2019 Nov;188:107794. doi: 10.1016/j.exer.2019.107794. Epub 2019 Sep 10. Exp Eye Res. 2019. PMID: 31518569 Review.
-
In Vitro Topographical Model of Fuchs Dystrophy for Evaluation of Corneal Endothelial Cell Monolayer Formation.Adv Healthc Mater. 2016 Nov;5(22):2896-2910. doi: 10.1002/adhm.201600848. Epub 2016 Oct 4. Adv Healthc Mater. 2016. PMID: 27701826
Cited by
-
Topical Ripasudil for the Treatment of Primary Corneal Endothelial Degeneration in Dogs.Transl Vis Sci Technol. 2022 Sep 1;11(9):2. doi: 10.1167/tvst.11.9.2. Transl Vis Sci Technol. 2022. PMID: 36048012 Free PMC article.
-
Design principles and therapeutic applications of novel synthetic WNT signaling agonists.iScience. 2024 May 8;27(6):109938. doi: 10.1016/j.isci.2024.109938. eCollection 2024 Jun 21. iScience. 2024. PMID: 38832011 Free PMC article. Review.
-
Ocular injury progression and cornea histopathology from chloropicrin vapor exposure: Relevant clinical biomarkers in mice.Exp Eye Res. 2023 May;230:109440. doi: 10.1016/j.exer.2023.109440. Epub 2023 Mar 17. Exp Eye Res. 2023. PMID: 36933694 Free PMC article.
-
Transpupillary-Guided Trans-Scleral Transplantation of Subretinal Grafts in a Retinal Degeneration Mouse Model.J Vis Exp. 2024 Jan 26;(203):10.3791/65448. doi: 10.3791/65448. J Vis Exp. 2024. PMID: 38345250 Free PMC article.
-
Early Visibility of Cellular Aggregates and Changes in Central Corneal Thickness as Predictors of Successful Corneal Endothelial Cell Injection Therapy.Cells. 2023 Apr 15;12(8):1167. doi: 10.3390/cells12081167. Cells. 2023. PMID: 37190076 Free PMC article.
References
-
- Damala M, Singh V. Corneal regeneration: Therapy and surgery. In: Alio JL, Alio del Barrio JL, Arnalich-Montiel F. editors. Corneal Regeneration: Therapy and Surgery. Cham: Springer, 2019:13-22.
