The role of neuroimaging in Parkinson's disease
- PMID: 34532856
- PMCID: PMC9291628
- DOI: 10.1111/jnc.15516
The role of neuroimaging in Parkinson's disease
Abstract
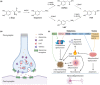
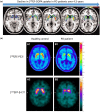
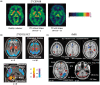
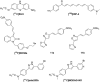
Parkinson's disease (PD) is a neurodegenerative disorder that affects millions of people worldwide. Two hallmarks of PD are the accumulation of alpha-synuclein and the loss of dopaminergic neurons in the brain. There is no cure for PD, and all existing treatments focus on alleviating the symptoms. PD diagnosis is also based on the symptoms, such as abnormalities of movement, mood, and cognition observed in the patients. Molecular imaging methods such as magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), and positron emission tomography (PET) can detect objective alterations in the neurochemical machinery of the brain and help diagnose and study neurodegenerative diseases. This review addresses the application of functional MRI, PET, and SPECT in PD patients. We provide an overview of the imaging targets, discuss the rationale behind target selection, the agents (tracers) with which the imaging can be performed, and the main findings regarding each target's state in PD. Molecular imaging has proven itself effective in supporting clinical diagnosis of PD and has helped reveal that PD is a heterogeneous disorder, which has important implications for the development of future therapies. However, the application of molecular imaging for early diagnosis of PD or for differentiation between PD and atypical parkinsonisms has remained challenging. The final section of the review is dedicated to new imaging targets with which one can detect the PD-related pathological changes upstream from dopaminergic degeneration. The foremost of those targets is alpha-synuclein. We discuss the progress of tracer development achieved so far and challenges on the path toward alpha-synuclein imaging in humans.
Keywords: PET; Parkinson's disease; SPECT; alpha-synuclein; neurodegeneration; neuroimaging.
© 2021 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Similar articles
-
Imaging in Parkinson's Disease.Int Rev Neurobiol. 2017;132:233-274. doi: 10.1016/bs.irn.2017.02.015. Epub 2017 Mar 30. Int Rev Neurobiol. 2017. PMID: 28554409 Review.
-
Translational molecular imaging and drug development in Parkinson's disease.Mol Neurodegener. 2023 Feb 10;18(1):11. doi: 10.1186/s13024-023-00600-z. Mol Neurodegener. 2023. PMID: 36759912 Free PMC article. Review.
-
Roles of molecular neuroimaging techniques in Parkinsonism.Discoveries (Craiova). 2023 Dec 31;11(4):e177. doi: 10.15190/d.2023.16. eCollection 2023 Oct-Dec. Discoveries (Craiova). 2023. PMID: 39529657 Free PMC article. Review.
-
Positron Emission Tomography Imaging of Synaptic Dysfunction in Parkinson's Disease.Neurosci Bull. 2024 Jun;40(6):743-758. doi: 10.1007/s12264-024-01188-0. Epub 2024 Mar 14. Neurosci Bull. 2024. PMID: 38483697 Free PMC article. Review.
-
Contributions of PET and SPECT to the understanding of the pathophysiology of Parkinson's disease.Neurophysiol Clin. 2001 Oct;31(5):321-40. doi: 10.1016/s0987-7053(01)00273-8. Neurophysiol Clin. 2001. PMID: 11817273 Review.
Cited by
-
Neuroimaging-based data-driven subtypes of spatiotemporal atrophy due to Parkinson's disease.Brain Commun. 2025 Apr 16;7(2):fcaf146. doi: 10.1093/braincomms/fcaf146. eCollection 2025. Brain Commun. 2025. PMID: 40303603 Free PMC article.
-
Precision Imaging in Neurodegeneration: The Superiority of Diffusion Tensor Imaging Over Conventional MRI in Differentiating Parkinson's Disease From Atypical Parkinsonian Syndromes.Cureus. 2024 Sep 8;16(9):e68933. doi: 10.7759/cureus.68933. eCollection 2024 Sep. Cureus. 2024. PMID: 39381485 Free PMC article.
-
The Association Between Neurocognitive Disorders and Gustatory Dysfunction: A Systematic Review and Meta-Analysis.Neuropsychol Rev. 2024 Mar;34(1):192-213. doi: 10.1007/s11065-023-09578-3. Epub 2023 Feb 20. Neuropsychol Rev. 2024. PMID: 36806051 Free PMC article.
-
PET, SPECT, and MRI imaging for evaluation of Parkinson's disease.Am J Nucl Med Mol Imaging. 2024 Dec 15;14(6):371-390. doi: 10.62347/AICM8774. eCollection 2024. Am J Nucl Med Mol Imaging. 2024. PMID: 39840378 Free PMC article. Review.
-
Evaluation of damage discrimination in dopaminergic neurons using dopamine transporter PET tracer [18F]FECNT-d4.EJNMMI Res. 2024 Aug 29;14(1):78. doi: 10.1186/s13550-024-01140-3. EJNMMI Res. 2024. PMID: 39210186 Free PMC article.
References
-
- Ametamey, S. M. , Honer, M. , & Schubiger, P. A. (2008). Molecular imaging with PET. Chemical Reviews, 108, 1501–1516. - PubMed
-
- Andersen, K. B. , Hansen, A. K. , Damholdt, M. F. , Horsager, J. , Skjærbæk, C. , Gottrup, H. , Klit, H. , Schacht, A. C. , Danielsen, E. H. , Brooks, D. J. , & Borghammer, P. (2021). Reduced synaptic density in patients with lewy body dementia: An [11C] UCB‐J PET imaging study. Movement Disorders, 36, 2057–2065. 10.1002/mds.28617. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

