Glutathione Infusion Before and 3 Days After Primary Angioplasty Blunts Ongoing NOX2-Mediated Inflammatory Response
- PMID: 34533039
- PMCID: PMC8649545
- DOI: 10.1161/JAHA.120.020560
Glutathione Infusion Before and 3 Days After Primary Angioplasty Blunts Ongoing NOX2-Mediated Inflammatory Response
Abstract
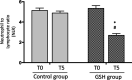
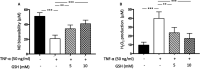
Background Glutathione is a water-soluble tripeptide with a potent oxidant scavenging activity. We hypothesized that glutathione administration immediately before and after primary angioplasty (primary percutaneous coronary intervention) could be effective in modulating immune cell activation, thereby preventing infarct expansion. Methods and Results One hundred consecutive patients with ST-segment-elevation myocardial infarction, scheduled to undergo primary percutaneous coronary intervention were randomly assigned before the intervention to receive an infusion of glutathione (2500 mg/25 mL over 10 minutes), followed by drug administration at the same doses at 24, 48, and 72 hours elapsing time or placebo. Total leukocytes, NOX2 (nicotinamide adenine dinucleotide phosphate oxidase 2) activation, NO bioavailability, cTpT (serum cardiac troponin T), hsCRP (high-sensitivity C-reactive protein), and TNF-α (tumor necrosis factor α) levels were measured. Left ventricular size and function were assessed within 120 minutes, 5 days, and 6 months from percutaneous coronary intervention. Following reperfusion, a significant reduction of neutrophil to lymphocyte ratio (P<0.0001), hsCRP generation (P<0.0001), NOX2 activation (P<0.0001), TNF-α levels (P<0.001), and cTpT release (P<0.0001) were found in the glutathione group compared with placebo. In treated patients, blunted inflammatory response was linked to better left ventricular size and function at follow-up (r=0.78, P<0.005). Conclusions Early and prolonged glutathione infusion seems able to protect vital myocardial components and endothelial cell function against harmful pro-oxidant and inflammatory environments, thus preventing maladaptive cardiac repair and left ventricular adverse remodeling. Registration URL: https://www.clinicaltrialsregister.eu; Unique identifier: 2014-004486-25.
Keywords: STEMI; immune cells; inflammation; left ventricular remodeling; oxidative stress; reperfusion injury.
Conflict of interest statement
None.
Figures

References
-
- Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, Mitamura H, Ogawa S. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: a possible role for left ventricular remodeling. J Am Coll Cardiol. 2002;39:241–246. DOI: 10.1016/S0735-1097(01)01721-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

