Angioscopic Evaluation of Atrial Septal Defect Closure Device Neo-Endothelialization
- PMID: 34533044
- PMCID: PMC8649546
- DOI: 10.1161/JAHA.120.019282
Angioscopic Evaluation of Atrial Septal Defect Closure Device Neo-Endothelialization
Abstract
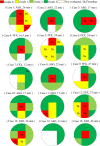
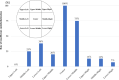
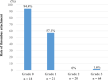
Background Current guidelines recommend at least 6 months of antithrombotic therapy and antibiotic prophylaxis after septal-occluding device deployment in transcatheter closure of atrial septal defect. It has been estimated that it takes ≈6 months for complete neo-endothelialization; however, neo-endothelialization has not previously been assessed in vivo in humans. Methods and Results The neointimal coverage of septal occluder devices was evaluated 6 months after implantation in 15 patients by angioscopy from the right atrium. Each occluder surface was divided into 9 areas; the levels of endothelialization in each area were semiquantitatively assessed by 4-point grades. Device neo-endothelialization was sufficient in two thirds of patients, but insufficient in one third. In the comparison between patients with sufficiently endothelialized devices of average grade score ≥2 (good endothelialization group, n=10) and those with poorly endothelialized devices of average grade score <2 (poor endothelialization group, n=5), those in the poor endothelialization group had larger devices deployed (27.0 mm [25.0-31.5 mm] versus 17.0 mm [15.6-22.5 mm], respectively) and progressive right heart dilatation. The endothelialization was poorer around the central areas. Moreover, the prevalence of thrombus formation on the devices was higher in the poorly endothelialized areas than in the sufficiently endothelialized areas (Grade 0, 94.1%; Grade 1, 63.2%; Grade 2, 0%; Grade 3, 1.6%). Conclusions Neo-endothelialization on the closure devices varied 6 months after implantation. Notably, poor endothelialization and thrombus attachment were observed around the central areas and on the larger devices.
Keywords: angioscopy; atrial septal defect; device neo‐endothelialization.
Conflict of interest statement
None.
Figures


References
-
- King TD, Mills NL. Nonoperative closure of atrial septal defects. Surgery. 1974;75:383–388. - PubMed
-
- Kutty S, Hazeem AA, Brown K, Danford CJ, Worley SE, Delaney JW, Danford DA, Latson LA. Long‐term (5‐ to 20‐year) outcomes after transcatheter or surgical treatment of hemodynamically significant isolated secundum atrial septal defect. Am J Cardiol. 2012;109:1348–1352. DOI: 10.1016/j.amjcard.2011.12.031. - DOI - PubMed
-
- Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. 2004;43:302–309. DOI: 10.1016/j.jacc.2003.10.030. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

