Evolving Diagnostic Criteria for Arrhythmogenic Cardiomyopathy
- PMID: 34533054
- PMCID: PMC8649536
- DOI: 10.1161/JAHA.121.021987
Evolving Diagnostic Criteria for Arrhythmogenic Cardiomyopathy
Abstract
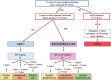
Criteria for diagnosis of arrhythmogenic cardiomyopathy (ACM) were first proposed in 1994 and revised in 2010 by a Task Force. Although the Task Force criteria demonstrated a good accuracy for diagnosis of the original right ventricular phenotype (arrhythmogenic right ventricular cardiomyopathy), they lacked sensitivity for identification of the expanding phenotypic spectrum of ACM, which includes left-sided variants and did not incorporate late-gadolinium enhancement findings by cardiac magnetic resonance. The 2020 International criteria ("Padua criteria") have been developed by International experts with the aim to improve the diagnosis of ACM by providing new criteria for the diagnosis of left ventricular phenotypic features. The key upgrade was the incorporation of tissue characterization findings by cardiac magnetic resonance for noninvasive detection of late-gadolinium enhancement/myocardial fibrosis that are determinants for characterization of arrhythmogenic biventricular and left ventricular cardiomyopathy. The 2020 International criteria are heavily dependent on cardiac magnetic resonance, which has become mandatory to characterize the ACM phenotype and to exclude other diagnoses. New criteria regarding left ventricular depolarization and repolarization ECG abnormalities and ventricular arrhythmias of left ventricular origin were also provided. This article reviews the evolving approach to diagnosis of ACM, going back to the 1994 and 2010 International Task Force criteria and then grapple with the modern 2020 International criteria.
Keywords: cardiac magnetic resonance; cardiomyopathy; diagnosis; sudden death; ventricular arrhythmia.
Conflict of interest statement
None.
Figures



References
-
- Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, Basso C, Bauce B, Brunckhorst C, Bucciarelli‐Ducci C, International Experts , et al. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J. 2020;41:1414–1429. DOI: 10.1093/eurheartj/ehz669. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

