Clinical and Molecular Findings After Autologous Stem Cell Transplantation or Cyclophosphamide for Scleroderma: Handling Missing Longitudinal Data
- PMID: 34533286
- PMCID: PMC8926930
- DOI: 10.1002/acr.24785
Clinical and Molecular Findings After Autologous Stem Cell Transplantation or Cyclophosphamide for Scleroderma: Handling Missing Longitudinal Data
Abstract
Objective: Among individuals with systemic sclerosis (SSc) randomized to cyclophosphamide (CYC) (n = 34) or hematopoietic stem cell transplantation (HSCT) (n = 33), we examined longitudinal trends of clinical, pulmonary function, and quality of life measures while accounting for the influence of early failures on treatment comparisons.
Methods: Assuming that data were missing at random, mixed-effects regression models were used to estimate longitudinal trends for clinical measures when comparing treatment groups. Results were compared to observed means and to longitudinal trends estimated from shared parameter models, assuming that data were missing not at random. Longitudinal trends for SSc intrinsic molecular subsets defined by baseline gene expression signatures (normal-like, inflammatory, and fibroproliferative signatures) were also studied.
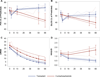
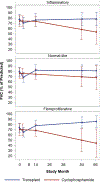
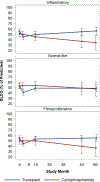
Results: Available observed means for pulmonary function tests appeared to improve over time in both arms. However, after accounting for participant loss, forced vital capacity in HSCT recipients increased by 0.77 percentage points/year but worsened by -3.70/year for CYC (P = 0.004). Similar results were found for diffusing capacity for carbon monoxide and quality of life indicators. Results for both analytic models were consistent. HSCT recipients in the inflammatory (n = 20) and fibroproliferative (n = 20) subsets had superior long-term trends compared to CYC for pulmonary and quality of life measures. HSCT was also superior for modified Rodnan skin thickness scores in the fibroproliferative subset. For the normal-like subset (n = 22), superiority of HSCT was less apparent.
Conclusion: Longitudinal trends estimated from 2 statistical models affirm the efficacy of HSCT over CYC in severe SSc. Failure to account for early loss of participants may distort estimated clinical trends over the long term.
© 2021 American College of Rheumatology.
Conflict of interest statement
Figures

References
-
- Mayes MD, Lacey JV Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheumatol 2003; 48:2246–2255. - PubMed
-
- Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2012; 51:1017–1026. - PubMed
-
- Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014; 6:1625–1635. - PubMed

