Heart failure in COVID-19: the multicentre, multinational PCHF-COVICAV registry
- PMID: 34533287
- PMCID: PMC8653014
- DOI: 10.1002/ehf2.13549
Heart failure in COVID-19: the multicentre, multinational PCHF-COVICAV registry
Abstract
Aims: We assessed the outcome of hospitalized coronavirus disease 2019 (COVID-19) patients with heart failure (HF) compared with patients with other cardiovascular disease and/or risk factors (arterial hypertension, diabetes, or dyslipidaemia). We further wanted to determine the incidence of HF events and its consequences in these patient populations.
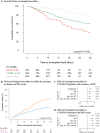
Methods and results: International retrospective Postgraduate Course in Heart Failure registry for patients hospitalized with COVID-19 and CArdioVascular disease and/or risk factors (arterial hypertension, diabetes, or dyslipidaemia) was performed in 28 centres from 15 countries (PCHF-COVICAV). The primary endpoint was in-hospital mortality. Of 1974 patients hospitalized with COVID-19, 1282 had cardiovascular disease and/or risk factors (median age: 72 [interquartile range: 62-81] years, 58% male), with HF being present in 256 [20%] patients. Overall in-hospital mortality was 25% (n = 323/1282 deaths). In-hospital mortality was higher in patients with a history of HF (36%, n = 92) compared with non-HF patients (23%, n = 231, odds ratio [OR] 1.93 [95% confidence interval: 1.44-2.59], P < 0.001). After adjusting, HF remained associated with in-hospital mortality (OR 1.45 [95% confidence interval: 1.01-2.06], P = 0.041). Importantly, 186 of 1282 [15%] patients had an acute HF event during hospitalization (76 [40%] with de novo HF), which was associated with higher in-hospital mortality (89 [48%] vs. 220 [23%]) than in patients without HF event (OR 3.10 [2.24-4.29], P < 0.001).
Conclusions: Hospitalized COVID-19 patients with HF are at increased risk for in-hospital death. In-hospital worsening of HF or acute HF de novo are common and associated with a further increase in in-hospital mortality.
Keywords: COVID-19; Cardiovascular disease; Heart failure; Risk factors; SARS-CoV2.
© 2021 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Sander Trenson: travel grants from Abbott, Daiichi Sankyo, and Boston Scientific; speaker fees from Novartis and Boehringer Ingelheim. Nana K. Poku: travel grants from Servier, Vifor Pharma, and Boehringer Ingelheim; speaker fees from Servier. Tor Biering‐Sørensen: steering committee member of the Amgen financed GALACTIC‐HF trial; advisory board: Sanofi Pasteur and Amgen; speaker honorarium: Novartis and Sanofi Pasteur; research grant: GE Healthcare and Sanofi Pasteur. Tor Biering‐Sørensen, Mats C. Højbjerg Lassen, and Kristoffer G. Skaarup received funding for the current project from the Novo Nordisk Foundation. Eduardo Barge‐Caballero: travel grants from Lilly, Abbot, Novartis, and Rovi; advisory fees from Abbot, Novartis, Boehringer, AstraZeneca, and Vifor; speaker fees from Abbot, Pfizer, Rovi, Novartis, Boehringer, Servier, AstraZeneca, and Vifor; academic grant from Abbot for the PCHF 2016–2017 edition; research grant from the Fundación Mutua Madrileña to investigate a potential protective effect of statins on COVID‐19. Anne‐Catherine Pouleur: advisory board/speaker fee from Astra‐Zeneca, MSD, Bayer, Novartis, Actelion, and Pfizer. Judith Schwaiger: travel grants from Amgen and Bayer. Stephan Winnik: travel support through Servier, Daichi‐Sankyo, Boehringer Ingelheim, Abbott, Bayer, and Fehling Instruments; educational grant support through institution by Boehringer Ingelheim, Abbott, and Boston Scientific; consulting/speaker fees from Abbott, Boston Scientific, and Boehringer Ingelheim. Matthias Paul: consultant fees for lectures and advisory board participation from Novartis, Servier, Vifor, and AstraZeneca. Jérôme Costa: speaker fees from the following medical companies: Novartis, Servier, Amgen, and BMS; advisory board: Novartis Grand Est, Novonordisk, and Sanofi Genzyme. Nathan Mewton: consultant honoraria, research, and travel grants from Novartis, Bayer, and MSD. Carlos E.L. Montenegro: speaker fees from the following medical companies: Novartis, AstraZeneca, Merck, and Servier. Yuya Matsue is affiliated to a department endowed by Philips Respironics, ResMed, and Fukuda Denshi and received remuneration from Otsuka Pharmaceutical Co and Novartis Japan and a research grant from Otsuka Pharmaceutical Co. Michal Marchel: speakers fees from Bayer, Novartis, and Pfizer. Lampros K. Michalis: advisory boards: Bayer and Sanofi; honararia: Menarini, Novartis, Actelion, AstraZeneca, Pfizer, and Elpen; research grants: Elpen and Medtronic. Marcus Dörr: travel grants from Servier; speaker fees from Bayer, AstraZeneca, Daichii Sankyo, Fresenius Medical Care, and Novartis. Felix Schoenrath: remuneration, consultancy fees, and/or travel support from Medtronic GmbH, Abbott GmbH & Co. KG, and Cardiorentis AG and a research grant from Novartis Pharma GmbH. Frank Ruschitzka has been paid for the time spent as a committee member for clinical trials, advisory boards, other forms of consulting and lectures, or presentations. These payments were made directly to the University of Zurich, and no personal payments were received in relation to these trials or other activities. Andreas J. Flammer declares fees from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Fresenius, Imedos Systems, Medtronic, MSD, Mundipharma, Novartis, Pierre Fabre, Pfizer, Roche, Schwabe Pharma, Vifor, and Zoll, as well as grant support by Novartis, AstraZeneca, and Berlin Heart unrelated to this article. No other disclosures were reported.
Figures
References
-
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID‐19. Nat Med 2020; 26: 1017–1032. - PMC - PubMed
-
- Nägele MP, Barthelmes J, Ludovici V, Cantatore S, von Eckardstein A, Enseleit F, Lüscher TF, Ruschitzka F, Sudano I, Flammer AJ. Retinal microvascular dysfunction in heart failure. Eur Heart J 2018; 39: 47–56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

