Tumour-stroma ratio outperforms tumour budding as biomarker in colon cancer: a cohort study
- PMID: 34533595
- PMCID: PMC8589816
- DOI: 10.1007/s00384-021-04023-4
Tumour-stroma ratio outperforms tumour budding as biomarker in colon cancer: a cohort study
Abstract
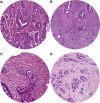
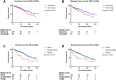
The tumour-stroma ratio (TSR) and tumour budding (TB) are two high-risk factors with potential to be implemented in the next TNM classification. The aim of the current study was to evaluate the practical application of the two biomarkers based on reproducibility, independency and prognostic value. Patients diagnosed with stage II or III colon cancer who underwent surgery between 2005 and 2016 were included. Both TSR and TB were scored on haematoxylin and eosin-stained tissue sections. The TSR, based on the relative amount of stroma, was scored in increments of 10%. TB was scored following the consensus guidelines; a bud was defined as ≤ 4 tumour cells. For analysis, three categories were used. Cohen's kappa was used for reproducibility. The prognostic value was determined with survival analysis. In total, 246 patients were included. The TSR distribution was N = 137 (56%) stroma-low and N = 109 (44%) stroma-high. The TB distribution was TB-low N = 194 (79%), TB-intermediate N = 35 (14%) and TB-high N = 17 (7%). The reproducibility of the TSR was good (interobserver agreement kappa = 0.83 and intraobserver agreement kappa = 0.82), whereas the inter- and intraobserver agreement for scoring TB was moderate (kappa 0.47 and 0.45, respectively). The survival analysis showed an independent prognostic value for disease-free survival for TSR (HR 1.57; 95% CI 1.01-2.44; p = 0.048) and for TB-high (HR 2.01; 95% CI 1.02-3.96; p = 0.043). Based on current results, we suggest the TSR is a more reliable parameter in daily practice due to better reproducibility and independent prognostic value for disease-free survival.
Keywords: Colon cancer; Personalised medicine; Tumour budding; Tumour microenvironment; Tumour-stroma ratio.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Aldecoa I, Atares B, Tarragona J, Bernet L, Sardon JD, Pereda T, Villar C, Mendez MC, Gonzalez-Obeso E, Elorriaga K, Alonso GL, Zamora J, Planell N, Palacios J, Castells A, Matias-Guiu X, Cuatrecasas M. Molecularly determined total tumour load in lymph nodes of stage I-II colon cancer patients correlates with high-risk factors. A multicentre prospective study. Virchows Arch. 2016;469:385–394. doi: 10.1007/s00428-016-1990-1. - DOI - PMC - PubMed
-
- Argiles G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.06.022. - DOI - PubMed
-
- Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, Brouwers M, Charette M, Haller DG. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. - DOI - PubMed
-
- Croner RS, Geppert CI, Bader FG, Nitsche U, Spath C, Rosenberg R, Zettl A, Matias-Guiu X, Tarragona J, Guller U, Sturzl M, Zuber M. Molecular staging of lymph node-negative colon carcinomas by one-step nucleic acid amplification (OSNA) results in upstaging of a quarter of patients in a prospective, European, Multicentre study. Br J Cancer. 2014;110:2544–2550. doi: 10.1038/bjc.2014.170. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

