Effect of a Multicomponent Sepsis Transition and Recovery Program on Mortality and Readmissions After Sepsis: The Improving Morbidity During Post-Acute Care Transitions for Sepsis Randomized Clinical Trial
- PMID: 34534130
- PMCID: PMC10229099
- DOI: 10.1097/CCM.0000000000005300
Effect of a Multicomponent Sepsis Transition and Recovery Program on Mortality and Readmissions After Sepsis: The Improving Morbidity During Post-Acute Care Transitions for Sepsis Randomized Clinical Trial
Abstract
Objectives: To evaluate whether a nurse navigator-led, multicomponent Sepsis Transition And Recovery program improves 30-day mortality and readmission outcomes after sepsis hospitalization.
Desig: n: Multisite pragmatic randomized clinical trial.
Setting: Three hospitals in North Carolina from January 2019 to March 2020.
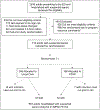
Patients: Eligible patients hospitalized for suspected sepsis and deemed high-risk for mortality or readmission by validated internal risk models.
Interventions: Patients were randomized to receive usual care alone (i.e., routine transition support, outpatient care; n = 342) or additional Sepsis Transition And Recovery support (n = 349). The 30-day intervention involved a multicomponent transition service led by a nurse navigator through telephone and electronic health record communication to facilitate best practice postsepsis care strategies during and after hospitalization including: postdischarge medication review, evaluation for new impairments or symptoms, monitoring comorbidities, and palliative care approach when appropriate. Clinical oversight was provided by a Hospital Medicine Transition Services team.
Measurements and main results: The primary outcome was a composite of mortality or hospital readmission at 30 days. Logistic regression models were constructed to evaluate marginal and conditional odds ratios (adjusted for prognostic covariates: age, comorbidity, and organ dysfunction at enrollment). Among 691 randomized patients (mean age = 63.7 ± 15.1 yr; 52% female), a lower percentage of patients in the Sepsis Transition And Recovery group experienced the primary outcome compared with the usual care group (28.7% vs 33.3%; risk difference, 4.7%; odds ratio, 0.80; 95% CI, 0.58-1.11; adjusted odds ratio, 0.80; 95% CI, 0.64-0.98). There were 74 deaths (Sepsis Transition And Recovery: 33 [9.5%] vs usual care: 41 [12.0%]) and 155 rehospitalizations (Sepsis Transition And Recovery: 71 [20.3%] vs usual care: 84 [24.6%]).
Conclusions: In a multisite randomized clinical trial of patients hospitalized with sepsis, patients provided with a 30-day program using a nurse navigator to provide best practices for postsepsis care experienced a lower proportion of either mortality or rehospitalization within 30 days after discharge. Further research is needed to understand the contextual factors associated with successful implementation.
Copyright © 2021 by the Society of Critical Care Medicine and Wolters Kluwer Health, Inc. All Rights Reserved.
Conflict of interest statement
Dr. Taylor reports grant support from National Institutes of Health (NIH) outside of the submitted work. Dr. Kowalkowski reports grant support from NIH and Patient-Centered Outcomes Research Institute outside of the submitted work. Drs. Taylor and Kowalkowski received funding from the National Institute of Nursing Research and the National Library of Medicine. Drs. Rios and Hetherington received support for article research from the NIH. Drs. Rios and Kowalkowski disclosed work for hire. Dr. McWilliams’ institution received funding from The Heineman Foundation; he disclosed he is the co-founder and administrative member of iEnroll, LLC. Drs. McWilliams’s and Hetherington’s institutions received funding from the NIH. Dr. Russo received funding from Moderna. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
Comment in
-
The IMPACT of Transitional Care Management in Sepsis.Crit Care Med. 2022 Mar 1;50(3):525-527. doi: 10.1097/CCM.0000000000005336. Crit Care Med. 2022. PMID: 35191876 No abstract available.
References
-
- Fleischmann C, Scherag A Adhikari NK, et al.; International Forum of Acute Care Trialists: Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–272 - PubMed