Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy - naive adults with HIV-1 infection
- PMID: 34534138
- PMCID: PMC8654248
- DOI: 10.1097/QAD.0000000000003070
Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy - naive adults with HIV-1 infection
Abstract
Objective: To assess efficacy and safety of dolutegravir (DTG) + lamivudine (3TC) vs. DTG + tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) in treatment-naive adults with HIV-1 in the prespecified 144-week secondary analyses of GEMINI-1 and GEMINI-2.
Design: Identical, multicenter, phase III, randomized, non-inferiority studies (double-blind through 96 weeks).
Methods: Participants with HIV-1 RNA ≤500 000 copies/ml and no major viral resistance mutations to nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, or protease inhibitors were randomized 1:1 to once-daily DTG + 3TC or DTG + TDF/FTC.
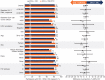
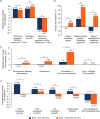
Results: At week 144, DTG + 3TC (N = 716) was noninferior to DTG + TDF/FTC (N = 717) in proportion of participants achieving HIV-1 RNA <50 copies/ml (Snapshot algorithm) in the pooled analysis (82% vs. 84%, respectively; adjusted treatment difference [95% confidence interval (CI)], -1.8% [-5.8, 2.1]), GEMINI-1 (-3.6% [-9.4, 2.1]), and GEMINI-2 (0.0% [-5.3, 5.3]). Twelve DTG + 3TC participants and nine DTG + TDF/FTC participants met protocol-defined confirmed virologic withdrawal (CVW) criteria; none developed treatment-emergent resistance. One DTG + 3TC participant who did not meet CVW criteria developed M184V at week 132 and R263R/K at week 144, conferring a 1.8-fold change in susceptibility to DTG; non-adherence to therapy was reported. Significantly fewer drug-related adverse events occurred with DTG + 3TC vs. DTG + TDF/FTC (20% vs. 27%; relative risk [95% CI], 0.76 [0.63-0.92]). Renal and bone biomarker changes favored DTG + 3TC.
Conclusions: Three-year durable efficacy, long-term tolerability, and high barrier to resistance support first-line use of DTG + 3TC for HIV-1 treatment (see Supplemental Digital Content 1, http://links.lww.com/QAD/C297; video abstract).
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
P.C. has served on advisory boards for GlaxoSmithKline (GSK), ViiV Healthcare, and Merck; served as an investigator for Abbott, Gilead, ViiV Healthcare, GSK, Merck, and Richmond; and has received honoraria for his speaking or chairing engagements from Abbott, GSK, Gilead, Merck, and ViiV Healthcare. J.S.M. has received lecture fees, sponsorship, and honoraria from Gilead, Stendhal, AbbVie, ViiV Healthcare, Janssen, and Merck Sharp & Dohme (MSD; all before 2019). J.R.A. has received advisory fees, speaker fees, and grant support from ViiV Healthcare, Janssen, Gilead, MSD, Alexa, and Teva. A.A. has served as a paid consultant to Gilead, Janssen, Merck, and ViiV Healthcare and received research funding from Gilead, Janssen, and ViiV Healthcare. R.O. has nothing to disclose. A.E.C. has received advisory fees from GSK, ViiV Healthcare, and Gilead; conference sponsorship from Gilead and Janssen; and speaker travel fees from GSK. C.-C.H. has received honoraria for speaking at educational events or consulting from AbbVie, Bristol-Myers Squibb (BMS), Gilead, Janssen, and ViiV Healthcare and has received research funding from BMS, Janssen, Gilead, Merck, and ViiV Healthcare. J.K.R. has received grant/research support from Gilead; served as a consultant/advisor to Abbott, AbbVie, Bionor, Gilead, Hexal, Janssen, Merck, and ViiV Healthcare; and was a speaker at educational events for AbbVie, Gilead, Janssen, and Merck. P.-M.G. has received grants from BMS and Janssen and has received honoraria and consulting fees from Gilead, ViiV Healthcare, and AbbVie. J.S., C.Y.M., M.U., K.A.P., K.Y.S., M.G., M.A., J.vW., and B.W. are employees of ViiV Healthcare and own stock in GSK. R.U., D.J.B., and L.C. are employees of GSK and own stock in GSK.
Author contributions: P.C., J.S., K.A.P., K.Y.S., M.G., M.A., and B.W. contributed to the conception of the study. P.C., J.S., R.U., M.U., K.A.P., K.Y.S., M.G., M.A., and B.W. contributed to the design of the study. P.C., J.S.M., J.R.A., A.A., R.O., A.E.C., C.-C.H., J.K.R., and P.-M.G. contributed to the acquisition of data. J.S., C.Y.M., R.U., D.J.B., M.U., L.C., and B.W. contributed to the analysis of data. P.C., J.S., C.Y.M., R.U., D.J.B., M.U., L.C., K.Y.S., J.vW., and B.W. contributed to the interpretation of data. J.S., C.Y.M., R.U., M.U., J.vW., and B.W. contributed to drafting the manuscript. All authors contributed to critically revising the manuscript for important intellectual content and approve the manuscript for publication.
Data sharing: Anonymized individual participant data and study documents can be requested for further research from
Figures

References
-
- Brenner BG, Wainberg MA. Clinical benefit of dolutegravir in HIV-1 management related to the high genetic barrier to drug resistance. Virus Res 2017; 239:1–9. - PubMed
-
- Taiwo BO, Zheng L, Stefanescu A, Nyaku A, Bezins B, Wallis CL, et al. . ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants with HIV-1 RNA <500000 copies/mL. Clin Infect Dis 2018; 66:1689–1697. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

