In vitro selection of Remdesivir resistance suggests evolutionary predictability of SARS-CoV-2
- PMID: 34534263
- PMCID: PMC8496873
- DOI: 10.1371/journal.ppat.1009929
In vitro selection of Remdesivir resistance suggests evolutionary predictability of SARS-CoV-2
Abstract
Remdesivir (RDV), a broadly acting nucleoside analogue, is the only FDA approved small molecule antiviral for the treatment of COVID-19 patients. To date, there are no reports identifying SARS-CoV-2 RDV resistance in patients, animal models or in vitro. Here, we selected drug-resistant viral populations by serially passaging SARS-CoV-2 in vitro in the presence of RDV. Using high throughput sequencing, we identified a single mutation in RNA-dependent RNA polymerase (NSP12) at a residue conserved among all coronaviruses in two independently evolved populations displaying decreased RDV sensitivity. Introduction of the NSP12 E802D mutation into our SARS-CoV-2 reverse genetics backbone confirmed its role in decreasing RDV sensitivity in vitro. Substitution of E802 did not affect viral replication or activity of an alternate nucleoside analogue (EIDD2801) but did affect virus fitness in a competition assay. Analysis of the globally circulating SARS-CoV-2 variants (>800,000 sequences) showed no evidence of widespread transmission of RDV-resistant mutants. Surprisingly, we observed an excess of substitutions in spike at corresponding sites identified in the emerging SARS-CoV-2 variants of concern (i.e., H69, E484, N501, H655) indicating that they can arise in vitro in the absence of immune selection. The identification and characterisation of a drug resistant signature within the SARS-CoV-2 genome has implications for clinical management and virus surveillance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


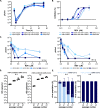
Comment in
-
Sars-Cov-2 and risk of antiviral drug resistance.Ir J Med Sci. 2022 Oct;191(5):2367-2368. doi: 10.1007/s11845-021-02820-y. Epub 2021 Oct 29. Ir J Med Sci. 2022. PMID: 34714491 Free PMC article. No abstract available.
References
-
- WHO Coronavirus (COVID-19) Dashboard. [cited 21 Jul 2021]. Available: https://covid19.who.int
-
- Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295: 6785–6797. doi: 10.1074/jbc.RA120.013679 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

