Conditional immobilization for live imaging Caenorhabditis elegans using auxin-dependent protein depletion
- PMID: 34534266
- PMCID: PMC8527506
- DOI: 10.1093/g3journal/jkab310
Conditional immobilization for live imaging Caenorhabditis elegans using auxin-dependent protein depletion
Abstract
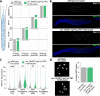
The visualization of biological processes using fluorescent proteins and dyes in living organisms has enabled numerous scientific discoveries. The nematode Caenorhabditis elegans is a widely used model organism for live imaging studies since the transparent nature of the worm enables imaging of nearly all tissues within a whole, intact animal. While current techniques are optimized to enable the immobilization of hermaphrodite worms for live imaging, many of these approaches fail to successfully restrain the smaller male worms. To enable live imaging of worms of both sexes, we developed a new genetic, conditional immobilization tool that uses the auxin-inducible degron (AID) system to immobilize both adult and larval hermaphrodite and male worms for live imaging. Based on chromosome location, mutant phenotype, and predicted germline consequence, we identified and AID-tagged three candidate genes (unc-18, unc-104, and unc-52). Strains with these AID-tagged genes were placed on auxin and tested for mobility and germline defects. Among the candidate genes, auxin-mediated depletion of UNC-18 caused significant immobilization of both hermaphrodite and male worms that was also partially reversible upon removal from auxin. Notably, we found that male worms require a higher concentration of auxin for a similar amount of immobilization as hermaphrodites, thereby suggesting a potential sex-specific difference in auxin absorption and/or processing. In both males and hermaphrodites, depletion of UNC-18 did not largely alter fertility, germline progression, nor meiotic recombination. Finally, we demonstrate that this new genetic tool can successfully immobilize both sexes enabling live imaging studies of sexually dimorphic features in C. elegans.
Keywords: C. elegans; auxin-inducible degron system; gametogenesis; germ cell development; germline; live imaging; meiosis; oogenesis; spermatogenesis; worms.
© The Author(s) 2021. Published by Oxford University Press on behalf of Genetics Society of America.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
