Survival and associated comorbidities in inclusion body myositis
- PMID: 34534271
- PMCID: PMC9071572
- DOI: 10.1093/rheumatology/keab716
Survival and associated comorbidities in inclusion body myositis
Erratum in
-
Correction to: Survival and associated comorbidities in inclusion body myositis.Rheumatology (Oxford). 2022 Nov 2;61(11):4580. doi: 10.1093/rheumatology/keac211. Rheumatology (Oxford). 2022. PMID: 35396988 Free PMC article. No abstract available.
Abstract
Objective: To evaluate survival and associated comorbidities in inclusion body myositis (IBM) in a population-based, case-control study.
Methods: We utilized the expanded Rochester Epidemiology Project medical records-linkage system, including 27 counties in Minnesota and Wisconsin, to identify patients with IBM, other inflammatory myopathies (IIM), and age/sex-matched population-controls. We compared the frequency of various comorbidities and survival among groups.
Results: We identified 50 IBM patients, 65 IIM controls and 294 population controls. Dysphagia was most common in IBM (64%) patients. The frequency of neurodegenerative disorders (dementia/parkinsonism) and solid cancers was not different between groups. Rheumatoid arthritis was the most common rheumatic disease in all groups. A total of 36% of IBM patients had a peripheral neuropathy, 6% had Sjögren's syndrome and 10% had a haematologic malignancy. T-cell large granular lymphocytic leukaemia was only observed in the IBM group. None of the IBM patients had hepatitis B or C, or HIV. IBM patients were 2.7 times more likely to have peripheral neuropathy, 6.2 times more likely to have Sjögren's syndrome and 3.9 times more likely to have a haematologic malignancy than population controls. IBM was associated with increased mortality, with a 10-year survival of 36% from index, compared with 67% in IIM and 59% in population controls. Respiratory failure or pneumonia (44%) was the most common cause of death.
Conclusions: IBM is associated with lower survival, and higher frequency of peripheral neuropathy, Sjögren's syndrome and haematologic malignancies than the general population. Close monitoring of IBM-related complications is warranted.
Keywords: Sjögren’s syndrome; case-control study; inclusion body myositis; large granular lymphocytic leukemia; peripheral neuropathy.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
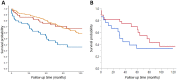
Comment in
-
Comment on: Survival and associated comorbidities in inclusion body myositis: Reply.Rheumatology (Oxford). 2022 Nov 2;61(11):e348-e349. doi: 10.1093/rheumatology/keac379. Rheumatology (Oxford). 2022. PMID: 35781321 No abstract available.
-
Comment on: Survival and associated comorbidities in inclusion body myositis.Rheumatology (Oxford). 2022 Nov 2;61(11):e346-e347. doi: 10.1093/rheumatology/keac378. Rheumatology (Oxford). 2022. PMID: 35781560 No abstract available.
References
-
- Greenberg SA. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol 2019;15:257–72. - PubMed
-
- Askanas V, Engel WK.. Inclusion-body myositis, a multifactorial muscle disease associated with aging: current concepts of pathogenesis. Curr Opin Rheumatol 2007;19:550–9. - PubMed
-
- Mendell JR, Sahenk Z, Gales T, Paul L.. Amyloid filaments in inclusion body myositis. Novel findings provide insight into nature of filaments. Arch Neurol 1991;48:1229–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

