Ciraparantag reverses the anticoagulant activity of apixaban and rivaroxaban in healthy elderly subjects
- PMID: 34534272
- PMCID: PMC8900496
- DOI: 10.1093/eurheartj/ehab637
Ciraparantag reverses the anticoagulant activity of apixaban and rivaroxaban in healthy elderly subjects
Abstract
Aims: Ciraparantag is a reversal agent for anticoagulants including direct oral anticoagulants. The aim was to evaluate the efficacy and safety of ciraparantag to reverse anticoagulation induced by apixaban or rivaroxaban in healthy elderly adults.
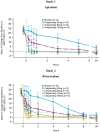
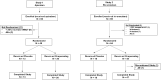
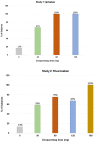
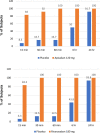
Methods and results: Two randomized, placebo-controlled, dose-ranging trials conducted in healthy subjects aged 50-75 years. Subjects received apixaban (Study 1) 10 mg orally twice daily for 3.5 days or rivaroxaban (Study 2) 20 mg orally once daily for 3 days. At steady-state anticoagulation subjects were randomized 3:1 to a single intravenous dose of ciraparantag (Study 1: 30, 60, or 120 mg; Study 2: 30, 60, 120, or 180 mg) or placebo. Efficacy was based on correction of the whole blood clotting time (WBCT) at multiple timepoints over 24 h. Subjects and technicians performing WBCT testing were blinded to treatment. Complete reversal of WBCT within 1 h post-dose and sustained through 5 h (apixaban) or 6 h (rivaroxaban) was dose related and observed with apixaban in 67%, 100%, 100%, and 17% of subjects receiving ciraparantag 30 mg, 60 mg, 120 mg, or placebo, respectively; and with rivaroxaban in 58%, 75%, 67%, 100%, and 13% of subjects receiving ciraparantag 30 mg, 60 mg, 120 mg, 180 mg, or placebo, respectively. Adverse events related to ciraparantag were mild, transient hot flashes or flushing.
Conclusions: Ciraparantag provides a dose-related reversal of anticoagulation induced by steady-state dosing of apixaban or rivaroxaban. Sustained reversal was achieved with 60 mg ciraparantag for apixaban and 180 mg ciraparantag for rivaroxaban. All doses of ciraparantag were well tolerated.
Keywords: Anticoagulant; Antidote; Apixaban; Ciraparantag; Rivaroxaban.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
Ciraparantag as a potential universal anticoagulant reversal agent.Eur Heart J. 2022 Mar 7;43(10):993-995. doi: 10.1093/eurheartj/ehab706. Eur Heart J. 2022. PMID: 34648621 No abstract available.
References
-
- Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbüchel H; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–1393. - PubMed
-
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JHRE, Wells P, Woller SC, Moores L. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–352. - PubMed
-
- Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, Hutten BA, Jaff MR, Manja V, Schulman S, Thurston C, Vedantham S, Verhamme P, Witt DM, Florez ID, Izcovich A, Nieuwlaat R, Ross S, Schunemann HJ, Wiercioch W, Zhang Y, Zhang Y. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 2020;4:4693–4738. - PMC - PubMed
-
- Niessner A, Tamargo J, Morais J, Koller L, Wassmann S, Husted SE, Torp-Pedersen C, Kjeldsen K, Lewis BS, Drexel H, Kaski JC, Atar D, Storey RF, Lip GYH, Verheugt FWA, Agewall S. Reversal strategies for non-vitamin K antagonist oral anticoagulants: a critical appraisal of available evidence and recommendations for clinical management—a joint position paper of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy and European Society of Cardiology Working Group on Thrombosis. Eur Heart J 2017;38:1710–1716. - PubMed

