Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection
- PMID: 34534287
- PMCID: PMC11506060
- DOI: 10.1093/eurheartj/ehab605
Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection
Erratum in
-
Corrigendum to: Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection.Eur Heart J. 2023 May 21;44(20):1844-1845. doi: 10.1093/eurheartj/ehad167. Eur Heart J. 2023. PMID: 37016271 No abstract available.
Abstract
Aims: Aortic aneurysm and dissection (AAD) are high-risk cardiovascular diseases with no effective cure. Macrophages play an important role in the development of AAD. As succinate triggers inflammatory changes in macrophages, we investigated the significance of succinate in the pathogenesis of AAD and its clinical relevance.
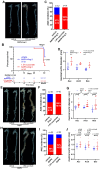
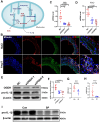
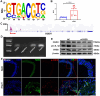
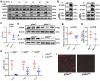
Methods and results: We used untargeted metabolomics and mass spectrometry to determine plasma succinate concentrations in 40 and 1665 individuals of the discovery and validation cohorts, respectively. Three different murine AAD models were used to determine the role of succinate in AAD development. We further examined the role of oxoglutarate dehydrogenase (OGDH) and its transcription factor cyclic adenosine monophosphate-responsive element-binding protein 1 (CREB) in the context of macrophage-mediated inflammation and established p38αMKOApoe-/- mice. Succinate was the most upregulated metabolite in the discovery cohort; this was confirmed in the validation cohort. Plasma succinate concentrations were higher in patients with AAD compared with those in healthy controls, patients with acute myocardial infarction (AMI), and patients with pulmonary embolism (PE). Moreover, succinate administration aggravated angiotensin II-induced AAD and vascular inflammation in mice. In contrast, knockdown of OGDH reduced the expression of inflammatory factors in macrophages. The conditional deletion of p38α decreased CREB phosphorylation, OGDH expression, and succinate concentrations. Conditional deletion of p38α in macrophages reduced angiotensin II-induced AAD.
Conclusion: Plasma succinate concentrations allow to distinguish patients with AAD from both healthy controls and patients with AMI or PE. Succinate concentrations are regulated by the p38α-CREB-OGDH axis in macrophages.
Keywords: Aortic aneurysm and dissection; Macrophage; Oxoglutarate dehydrogenase; Succinate.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures



Comment in
-
Breaking the cycle: Succinate in aortic diseases.Eur Heart J. 2021 Nov 7;42(42):4386-4388. doi: 10.1093/eurheartj/ehab514. Eur Heart J. 2021. PMID: 34564722 No abstract available.
References
-
- Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep 2011;59:1–126. - PubMed
-
- Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RSv, Vrints CJM. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–2926. - PubMed
-
- Guo DC, Papke CL, He R, Milewicz DM. Pathogenesis of thoracic and abdominal aortic aneurysms. Ann N Y Acad Sci 2006;1085:339–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

