Genetic screen for suppressors of increased silencing in rpd3 mutants in Saccharomyces cerevisiae identifies a potential role for H3K4 methylation
- PMID: 34534290
- PMCID: PMC8527511
- DOI: 10.1093/g3journal/jkab309
Genetic screen for suppressors of increased silencing in rpd3 mutants in Saccharomyces cerevisiae identifies a potential role for H3K4 methylation
Abstract
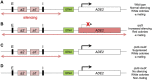
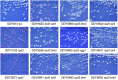
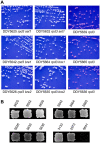
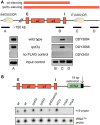
Several studies have identified the paradoxical phenotype of increased heterochromatic gene silencing at specific loci that results from deletion or mutation of the histone deacetylase (HDAC) gene RPD3. To further understand this phenomenon, we conducted a genetic screen for suppressors of this extended silencing phenotype at the HMR locus in Saccharomyces cerevisiae. Most of the mutations that suppressed extended HMR silencing in rpd3 mutants without completely abolishing silencing were identified in the histone H3 lysine 4 methylation (H3K4me) pathway, specifically in SET1, BRE1, and BRE2. These second-site mutations retained normal HMR silencing, therefore, appear to be specific for the rpd3Δ extended silencing phenotype. As an initial assessment of the role of H3K4 methylation in extended silencing, we rule out some of the known mechanisms of Set1p/H3K4me mediated gene repression by HST1, HOS2, and HST3 encoded HDACs. Interestingly, we demonstrate that the RNA Polymerase III complex remains bound and active at the HMR-tDNA in rpd3 mutants despite silencing extending beyond the normal barrier. We discuss these results as they relate to the interplay among different chromatin-modifying enzyme functions and the importance of further study of this enigmatic phenomenon.
Keywords: BRE1; BRE2; RPD3; SET1; Saccharomyces cerevisiae; COMPASS; chromatin; histone modifications; silencing.
© The Author(s) 2021. Published by Oxford University Press on behalf of Genetics Society of America.
Figures



References
-
- Allis CD, Jenuwein T.. 2016. The molecular hallmarks of epigenetic control. Nat Rev Genet. 17:487–500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
