Natural sensory context drives diverse brain-wide activity during C. elegans mating
- PMID: 34534446
- PMCID: PMC8488019
- DOI: 10.1016/j.cell.2021.08.024
Natural sensory context drives diverse brain-wide activity during C. elegans mating
Abstract
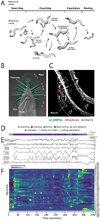
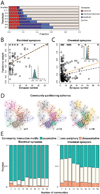
Natural goal-directed behaviors often involve complex sequences of many stimulus-triggered components. Understanding how brain circuits organize such behaviors requires mapping the interactions between an animal, its environment, and its nervous system. Here, we use brain-wide neuronal imaging to study the full performance of mating by the C. elegans male. We show that as mating unfolds in a sequence of component behaviors, the brain operates similarly between instances of each component but distinctly between different components. When the full sensory and behavioral context is taken into account, unique roles emerge for each neuron. Functional correlations between neurons are not fixed but change with behavioral dynamics. From individual neurons to circuits, our study shows how diverse brain-wide dynamics emerge from the integration of sensory perception and motor actions in their natural context.
Keywords: C. elegans; complex behavior; mating; neuroethology; systems neuroscience; whole brain imaging.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Adai AT, Date SV, Wieland S, and Marcotte EM (2004). Lgl: creating a map of protein function with an algorithm for visualizing very large biological networks. Journal of molecular biology 340, 179–190. - PubMed
-
- Ahn YY, Bagrow JP, and Lehmann S (2010). Link communities reveal multiscale complexity in networks. nature 466, 761–764. - PubMed
-
- Aicher C, Jacobs AZ, and Clauset A (2015). Learning latent block structure in weighted networks. Journal of Complex Networks 3, 221–248.
-
- Benjamini Y, and Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57, 289–300.
-
- Betzel RF, Bertolero MA, and Bassett DS (2018). Non-assortative community structure in resting and task-evoked functional brain networks. bioRxiv p. 355016.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

