Signature morpho-electric, transcriptomic, and dendritic properties of human layer 5 neocortical pyramidal neurons
- PMID: 34534454
- PMCID: PMC8570452
- DOI: 10.1016/j.neuron.2021.08.030
Signature morpho-electric, transcriptomic, and dendritic properties of human layer 5 neocortical pyramidal neurons
Abstract
In the neocortex, subcerebral axonal projections originate largely from layer 5 (L5) extratelencephalic-projecting (ET) neurons. The unique morpho-electric properties of these neurons have been mainly described in rodents, where retrograde tracers or transgenic lines can label them. Similar labeling strategies are infeasible in the human neocortex, rendering the translational relevance of findings in rodents unclear. We leveraged the recent discovery of a transcriptomically defined L5 ET neuron type to study the properties of human L5 ET neurons in neocortical brain slices derived from neurosurgeries. Patch-seq recordings, where transcriptome, physiology, and morphology were assayed from the same cell, revealed many conserved morpho-electric properties of human and rodent L5 ET neurons. Divergent properties were often subtler than differences between L5 cell types within these two species. These data suggest a conserved function of L5 ET neurons in the neocortical hierarchy but also highlight phenotypic divergence possibly related to functional specialization of human neocortex.
Keywords: cross-species; dendrite; dendritic spike; gene expression; human; intrinsic membrane properties; patch-clamp physiology; patch-seq; pyramidal neuron; transcriptomics.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.T.G., T.L.D., J.T.T., E.L., B.K., H.Z., and B.T. are inventors on a PCT application (PCT/US2019/059927) related to this work. All authors declare no other competing interests.
Figures


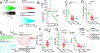

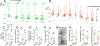


References
-
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, and Hof PR (2010). The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct. Funct 214, 495–517. - PubMed
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, and Macklis JD (2005). Neuronal Subtype-Specific Genes that Control Corticospinal Motor Neuron Development In Vivo. Neuron 45, 207–221. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

