BCG re-vaccination in Malawi: 30-year follow-up of a large, randomised, double-blind, placebo-controlled trial
- PMID: 34534489
- PMCID: PMC8459381
- DOI: 10.1016/S2214-109X(21)00309-0
BCG re-vaccination in Malawi: 30-year follow-up of a large, randomised, double-blind, placebo-controlled trial
Abstract
Background: A large, double-blind, randomised, placebo-controlled trial of repeat BCG found 49% efficacy against leprosy but no protection against tuberculosis after 6-9 years' follow-up in 1995. We report here additional follow-up, which resulted in greatly increased tuberculosis case numbers, and allowed subgroup analysis.
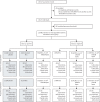
Methods: Nearly 47 000 individuals of all ages living in northern Malawi with a BCG vaccine scar were randomly assigned (1:1) between 1986 and 1989 to receive a second BCG or placebo. The investigators and project staff remained masked to all interventions. Enhanced passive surveillance ensured ascertainment of tuberculosis and leprosy to the end of 2018. Tuberculosis case definitions included rigorous microbiological or histological confirmation. Prespecified subgroup analyses were by tuberculosis type, age at vaccination, time since vaccination, previous tuberculin reactivity, HIV status and Mycobacterium tuberculosis lineage. The original trial is registered with ISRCTN registry, ISRCTN11311670.
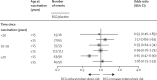
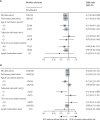
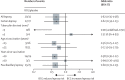
Findings: In follow-up until Dec 31, 2018, 824 participants had developed tuberculosis, including 786 with pulmonary disease, of whom 383 (63%) of 607 with known HIV status were HIV positive. There was no effect of a second BCG overall (odds ratio [OR] 0·92; 95% CI 0·80-1·05), or for pulmonary (0·93; 0·81-1·07), or lymph node tuberculosis (0·60; 0·31-1·17). The OR was lower for those with known HIV-negative tuberculosis (0·77; 0·59-1·00), for those vaccinated as children (aged <5 years, 0·74; 0·41-1·35; aged 5-14 years, 0·77; 0·60-0·99), and for cases arising at least 20 years after vaccination (0·79; 0·63-1·01). There were no differences by tuberculin status at vaccination, or lineage. There was no evidence of protection against leprosy beyond 10 years after vaccination (although there have been only nine diagnostically certain cases since 1995).
Interpretation: There was no evidence that repeat BCG vaccination provides appreciable protection against overall tuberculosis in this rural African population with a high prevalence of HIV. Subgroup effects should not be overinterpreted given the multiple analyses done. However, the evidence for modest protection against HIV-negative tuberculosis, and for a delayed benefit in those vaccinated as children, is consistent with other observations in the literature.
Funding: LEPRA, Wellcome Trust, Bill & Melinda Gates Foundation.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests JRG, KF, and PEMF received grant funding from the Gates Foundation for this work. All other authors declare no competing interests.
Figures

Comment on
-
Follow-up of a BCG trial after 30 years: what lessons have we learned?Lancet Glob Health. 2021 Oct;9(10):e1353-e1354. doi: 10.1016/S2214-109X(21)00339-9. Lancet Glob Health. 2021. PMID: 34534474 No abstract available.
References
-
- WHO–SAGE BCG vaccines: WHO position paper—February 2018. Wkly Epidemiol Rec. 2018;93:73–96. - PubMed
-
- Rodrigues LC, Pereira SM, Cunha SS. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005;366:1290–1295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

