Genetic profiles of subcutaneous panniculitis-like T-cell lymphoma and clinicopathological impact of HAVCR2 mutations
- PMID: 34535012
- PMCID: PMC8945616
- DOI: 10.1182/bloodadvances.2021004562
Genetic profiles of subcutaneous panniculitis-like T-cell lymphoma and clinicopathological impact of HAVCR2 mutations
Erratum in
-
Koh J, Jang I, Mun S, et al. Genetic profiles of subcutaneous panniculitis-like T-cell lymphoma and clinicopathological impact of HAVCR2 mutations. Blood Adv. 2021;5(20):3919-3930.Blood Adv. 2022 Jun 28;6(12):3659-3660. doi: 10.1182/bloodadvances.2022007177. Blood Adv. 2022. PMID: 35727606 Free PMC article. No abstract available.
Abstract
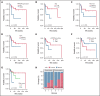
Recent studies identified germline mutations in HAVCR2 (encoding T-cell immunoglobulin mucin 3) as a genetic factor that predisposes to subcutaneous panniculitis-like T-cell lymphoma (SPTCL). However, the differences between HAVCR2-mutated (HAVCR2MUT) and HAVCR2 wild-type (HAVCR2WT) SPTCLs remain unclear. A nationwide cohort of 53 patients with SPTCL diagnosed at 8 Korean institutions was established. Whole-exome sequencing and RNA-sequencing were performed on 8 patients in the discovery set. In the validation set, targeted gene sequencing or direct sequencing of HAVCR2 was performed. Of 49 patients with available HAVCR2 status, 25 (51.0%) were HAVCR2Y82C. HAVCR2Y82C was associated with younger age (P = .001), development of hemophagocytic lymphohistiocytosis or hemophagocytic lymphohistiocytosis-like systemic illness (P < .001), and short relapse-free survival (RFS) (P = .023). Most mutated genes in SPTCLs were involved in immune responses, epigenetic modifications, and cell signaling. Mutations in UNC13D, PIAS3, and KMT2D were more frequent in HAVCR2WT SPTCLs. At the gene expression level, HAVCR2Y82C SPTCLs were enriched in genes involved in IL6-JAK-STAT3 signaling and in tumor necrosis factor-α signaling via NF-κB. CCR4 was significantly upregulated in HAVCR2WT SPTCLs both at the messenger RNA level and at the protein level. We established a risk stratification system for SPTCL by integrating clinical and histopathological features, including age and HAVCR2 mutation status. This risk stratification system was strongly associated with RFS (P = .031). In conclusion, the HAVCR2Y82C mutation was common in Korean patients with SPTCL and was associated with unique clinicopathological and genetic features. Combining clinicopathological parameters could aid in predicting prognosis for patients with SPTCL.
© 2021 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures




References
-
- Jung HR, Huh J, Ko Y-H, et al. . Classification of malignant lymphoma subtypes in Korean patients: a report of the 4th nationwide study. J Hematop. 2019;12(4):173-181.
-
- Willemze R, Jansen PM, Cerroni L, et al. EORTC Cutaneous Lymphoma Group . Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111(2):838-845. - PubMed
-
- Willemze R. Cutaneous lymphomas with a panniculitic presentation. Semin Diagn Pathol. 2017;34(1):36-43. - PubMed
-
- Gayden T, Sepulveda FE, Khuong-Quang D-A, et al. . Germline HAVCR2 mutations altering TIM-3 characterize subcutaneous panniculitis-like T cell lymphomas with hemophagocytic lymphohistiocytic syndrome [published correction appears in Nat Genet. 2019;51(1):196 ]. Nat Genet. 2018; 50(12):1650-1657. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

