Early versus late awake prone positioning in non-intubated patients with COVID-19
- PMID: 34535158
- PMCID: PMC8446738
- DOI: 10.1186/s13054-021-03761-9
Early versus late awake prone positioning in non-intubated patients with COVID-19
Abstract
Background: Awake prone positioning (APP) is widely used in the management of patients with coronavirus disease (COVID-19). The primary objective of this study was to compare the outcome of COVID-19 patients who received early versus late APP.
Methods: Post hoc analysis of data collected for a randomized controlled trial (ClinicalTrials.gov NCT04325906). Adult patients with acute hypoxemic respiratory failure secondary to COVID-19 who received APP for at least one hour were included. Early prone positioning was defined as APP initiated within 24 h of high-flow nasal cannula (HFNC) start. Primary outcomes were 28-day mortality and intubation rate.
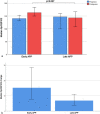
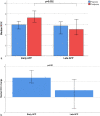
Results: We included 125 patients (79 male) with a mean age of 62 years. Of them, 92 (73.6%) received early APP and 33 (26.4%) received late APP. Median time from HFNC initiation to APP was 2.25 (0.8-12.82) vs 36.35 (30.2-75.23) hours in the early and late APP group (p < 0.0001), respectively. Average APP duration was 5.07 (2.0-9.05) and 3.0 (1.09-5.64) hours per day in early and late APP group (p < 0.0001), respectively. The early APP group had lower mortality compared to the late APP group (26% vs 45%, p = 0.039), but no difference was found in intubation rate. Advanced age (OR 1.12 [95% CI 1.0-1.95], p = 0.001), intubation (OR 10.65 [95% CI 2.77-40.91], p = 0.001), longer time to initiate APP (OR 1.02 [95% CI 1.0-1.04], p = 0.047) and hydrocortisone use (OR 6.2 [95% CI 1.23-31.1], p = 0.027) were associated with increased mortality.
Conclusions: Early initiation (< 24 h of HFNC use) of APP in acute hypoxemic respiratory failure secondary to COVID-19 improves 28-day survival. Trial registration ClinicalTrials.gov NCT04325906.
Keywords: Acute hypoxemic respiratory failure; Awake prone positioning; COVID-19; Coronavirus; Non-intubated.
© 2021. The Author(s).
Conflict of interest statement
Dr. Kaur disclose research funding from American Association of Respiratory Care. Dr. Vines discloses research funding from Teleflex Medical, Inc. and Rice Foundation, and speaker fees from Theravance Biopharma. Dr. Scott declares to receive research funding from Teleflex. Dr. Trump discloses consulting fees from Fisher and Paykel. Dr. Li discloses research funding from Fisher & Paykel Healthcare Ltd, Aerogen Ltd, and Rice Foundation, and speaker fees from American Association for Respiratory Care, Aerogen Ltd, and Fisher & Paykel Healthcare Ltd. Other authors have no conflict of interest to disclose.
Figures



Comment in
-
Early versus late proning in non-intubated COVID-19 pneumonia.Crit Care. 2021 Dec 10;25(1):422. doi: 10.1186/s13054-021-03838-5. Crit Care. 2021. PMID: 34893095 Free PMC article. No abstract available.
References
-
- World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report–1. Accessed March 22, 2021.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

