Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions
- PMID: 34535655
- PMCID: PMC8448846
- DOI: 10.1038/s41467-021-25585-5
Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions
Abstract
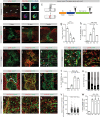
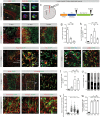
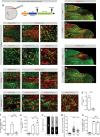
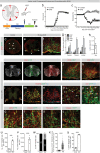
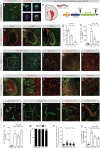
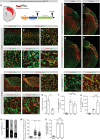
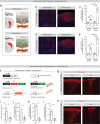
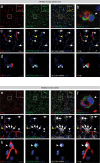
Fibrotic scar tissue limits central nervous system regeneration in adult mammals. The extent of fibrotic tissue generation and distribution of stromal cells across different lesions in the brain and spinal cord has not been systematically investigated in mice and humans. Furthermore, it is unknown whether scar-forming stromal cells have the same origin throughout the central nervous system and in different types of lesions. In the current study, we compared fibrotic scarring in human pathological tissue and corresponding mouse models of penetrating and non-penetrating spinal cord injury, traumatic brain injury, ischemic stroke, multiple sclerosis and glioblastoma. We show that the extent and distribution of stromal cells are specific to the type of lesion and, in most cases, similar between mice and humans. Employing in vivo lineage tracing, we report that in all mouse models that develop fibrotic tissue, the primary source of scar-forming fibroblasts is a discrete subset of perivascular cells, termed type A pericytes. Perivascular cells with a type A pericyte marker profile also exist in the human brain and spinal cord. We uncover type A pericyte-derived fibrosis as a conserved mechanism that may be explored as a therapeutic target to improve recovery after central nervous system lesions.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Göritz, C. et al. A pericyte origin of spinal cord scar tissue. Science333, 238–342 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

