Spatially and optically tailored 3D printing for highly miniaturized and integrated microfluidics
- PMID: 34535656
- PMCID: PMC8448845
- DOI: 10.1038/s41467-021-25788-w
Spatially and optically tailored 3D printing for highly miniaturized and integrated microfluidics
Abstract
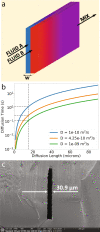
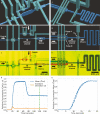
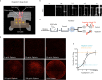
Traditional 3D printing based on Digital Light Processing Stereolithography (DLP-SL) is unnecessarily limiting as applied to microfluidic device fabrication, especially for high-resolution features. This limitation is due primarily to inherent tradeoffs between layer thickness, exposure time, material strength, and optical penetration that can be impossible to satisfy for microfluidic features. We introduce a generalized 3D printing process that significantly expands the accessible spatially distributed optical dose parameter space to enable the fabrication of much higher resolution 3D components without increasing the resolution of the 3D printer. Here we demonstrate component miniaturization in conjunction with a high degree of integration, including 15 μm × 15 μm valves and a 2.2 mm × 1.1 mm 10-stage 2-fold serial diluter. These results illustrate our approach's promise to enable highly functional and compact microfluidic devices for a wide variety of biomolecular applications.
© 2021. The Author(s).
Conflict of interest statement
Two of the authors (G.P.N. and A.T.W.) own shares in Acrea 3D, a company commercializing microfluidic 3D printing. The remaining authors declare no competing interests.
Figures




References
-
- Lee G-B, Chen S-H, Huang G-R, Sung W-C, Lin Y-H. Microfabricated plastic chips by hot embossing methods and their applications for dna separation and detection. Sens. Actuators B: Chem. 2001;75:142–148. doi: 10.1016/S0925-4005(00)00745-0. - DOI
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

