Emerging therapeutic opportunities for integrin inhibitors
- PMID: 34535788
- PMCID: PMC8446727
- DOI: 10.1038/s41573-021-00284-4
Emerging therapeutic opportunities for integrin inhibitors
Abstract
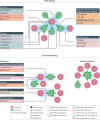
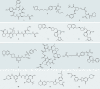
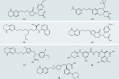
Integrins are cell adhesion and signalling proteins crucial to a wide range of biological functions. Effective marketed treatments have successfully targeted integrins αIIbβ3, α4β7/α4β1 and αLβ2 for cardiovascular diseases, inflammatory bowel disease/multiple sclerosis and dry eye disease, respectively. Yet, clinical development of others, notably within the RGD-binding subfamily of αv integrins, including αvβ3, have faced significant challenges in the fields of cancer, ophthalmology and osteoporosis. New inhibitors of the related integrins αvβ6 and αvβ1 have recently come to the fore and are being investigated clinically for the treatment of fibrotic diseases, including idiopathic pulmonary fibrosis and nonalcoholic steatohepatitis. The design of integrin drugs may now be at a turning point, with opportunities to learn from previous clinical trials, to explore new modalities and to incorporate new findings in pharmacological and structural biology. This Review intertwines research from biological, clinical and medicinal chemistry disciplines to discuss historical and current RGD-binding integrin drug discovery, with an emphasis on small-molecule inhibitors of the αv integrins.
© 2021. Springer Nature Limited.
Conflict of interest statement
R.J.D.H. and S.J.F.M. hold GSK shares and are applicants on GSK integrin patents. R.J.S. and J.A.R. hold GSK shares and are currently Galecto, Inc. employees and shareholders. R.G.J. reports grants from GlaxoSmithKline, grants and personal fees from Pliant Therapeutics, grants from Biogen, during the conduct of the study; personal fees from Galapagos, other from Galecto, personal fees and other from GlaxoSmithKline, personal fees and other from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Pliant, personal fees from Bristol-Myers Squibb, personal fees from Chiesi, personal fees from Roche/Promedior, personal fees and other from RedX, other from NuMedii, other from Nordic Biosciences, personal fees from Veracyte, personal fees from PatientMPower, personal fees from Resolution Therapeutics, personal fees from Vicore, outside the submitted work; he is supported by a National Institute of Health Research Professorship (NIHR ref: RP-2017-08-ST2-014). He is a trustee for Action for Pulmonary Fibrosis.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

