Prospective analysis of myocardial strain through the evolution of Chagas disease in the hamster animal model
- PMID: 34535853
- PMCID: PMC8818632
- DOI: 10.1007/s10554-021-02379-w
Prospective analysis of myocardial strain through the evolution of Chagas disease in the hamster animal model
Abstract
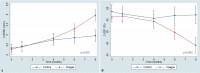
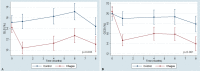
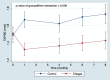
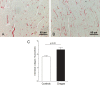
Speckle tracking echocardiography (STE) enables early diagnosis of myocardial damage by evaluating myocardial strain. We aimed to study sequential changes in structural and ventricular functional parameters during Chagas disease (CD) natural history in an animal model. 37 Syrian hamsters were inoculated intraperitoneally with Trypanosoma cruzi (Chagas) and 20 with saline (Control). Echocardiography was performed before the infection (baseline), at 1 month (acute phase), 4, 6, and 8 months (chronic phase) using Vevo 2100 (Fujifilm Inc.) ultrasound system. Left ventricular end-diastolic diameter, Left ventricular end-systolic diameter (LVESD), Left ventricular ejection fraction (LVEF), Global longitudinal (GLS), circumferential (GCS) and radial (GRS) strain were evaluated. Tricuspid annular plane systolic excursion (TAPSE) was used to assess right ventricular function. At 8 months, animals were euthanized and LV myocardial samples were analyzed for quantitation of inflammation and fibrosis. LVEF decreased over time in Chagas group and a difference from Control was detected at 6 months (p-value of groups#time interaction = 0.005). There was a pronounced decrease in GLS, GCS and TAPSE in Chagas group (p-value of groups#time interaction = 0.003 for GLS, < 0.001 for GCS and < 0.009 for TAPSE vs Control) since the first month. LVESD, LVEF and GLS were significantly correlated to the number of inflammatory cells (r = 0.41, p = 0.046; r = - 0.42, p = 0.042; r = 0.41, p = 0.047) but not to fibrosis. In the Syrian hamster model of CD STE parameters (GLS and GCS) showed an early decrease. Changes in LVEF, LVESD, and GLS were correlated to myocardial inflammation but not to fibrosis.
Keywords: Animal experimental model; Chagas cardiomyopathy; Echocardiography; Hamsters; Speckle-tracking; Strain.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Global Burden of Disease Study C Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

