The recurrent missense mutation p.(Arg367Trp) in YARS1 causes a distinct neurodevelopmental phenotype
- PMID: 34536092
- PMCID: PMC8599376
- DOI: 10.1007/s00109-021-02124-9
The recurrent missense mutation p.(Arg367Trp) in YARS1 causes a distinct neurodevelopmental phenotype
Erratum in
-
Correction to: The recurrent missense mutation p.(Arg367Trp) in YARS1 causes a distinct neurodevelopmental phenotype.J Mol Med (Berl). 2021 Dec;99(12):1769-1770. doi: 10.1007/s00109-021-02153-4. J Mol Med (Berl). 2021. PMID: 34661688 Free PMC article. No abstract available.
Abstract
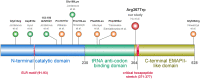
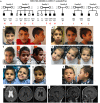
Pathogenic variants in aminoacyl-tRNA synthetases (ARS1) cause a diverse spectrum of autosomal recessive disorders. Tyrosyl tRNA synthetase (TyrRS) is encoded by YARS1 (cytosolic, OMIM*603,623) and is responsible of coupling tyrosine to its specific tRNA. Next to the enzymatic domain, TyrRS has two additional functional domains (N-Terminal TyrRSMini and C-terminal EMAP-II-like domain) which confer cytokine-like functions. Mutations in YARS1 have been associated with autosomal-dominant Charcot-Marie-Tooth (CMT) neuropathy type C and a heterogenous group of autosomal recessive, multisystem diseases. We identified 12 individuals from 6 families with the recurrent homozygous missense variant c.1099C > T;p.(Arg367Trp) (NM_003680.3) in YARS1. This variant causes a multisystem disorder with developmental delay, microcephaly, failure to thrive, short stature, muscular hypotonia, ataxia, brain anomalies, microcytic anemia, hepatomegaly, and hypothyroidism. In silico analyses show that the p.(Arg367Trp) does not affect the catalytic domain responsible of enzymatic coupling, but destabilizes the cytokine-like C-terminal domain. The phenotype associated with p.(Arg367Trp) is distinct from the other biallelic pathogenic variants that reside in different functional domains of TyrRS which all show some common, but also divergent clinical signs [(e.g., p.(Phe269Ser)-retinal anomalies, p.(Pro213Leu)/p.(Gly525Arg)-mild ID, p.(Pro167Thr)-high fatality)]. The diverse clinical spectrum of ARS1-associated disorders is related to mutations affecting the various non-canonical domains of ARS1, and impaired protein translation is likely not the exclusive disease-causing mechanism of YARS1- and ARS1-associated neurodevelopmental disorders. KEY MESSAGES: The missense variant p.(Arg367Trp) in YARS1 causes a distinct multisystem disorder. p.(Arg367Trp) affects a non-canonical domain with cytokine-like functions. Phenotypic heterogeneity associates with the different affected YARS1 domains. Impaired protein translation is likely not the exclusive mechanism of ARS1-associated disorders.
Keywords: Aminoacyl-tRNA synthetases (ARS1); Functional protein domains; Multisystem diseases; Neurodevelopmental disorders; Novel disease genes; Phenotypic heterogeneity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Kanaji T, Vo MN, Kanaji S, Zarpellon A, Shapiro R, Morodomi Y, Yuzuriha A, Eto K, Belani R, Do MH, et al. Tyrosyl-tRNA synthetase stimulates thrombopoietin-independent hematopoiesis accelerating recovery from thrombocytopenia. Proc Natl Acad Sci USA. 2018;115(35):E8228–E8235. doi: 10.1073/pnas.1807000115. - DOI - PMC - PubMed
-
- Kao J, Ryan J, Brett G, Chen J, Shen H, Fan YG, Godman G, Familletti PC, Wang F, Pan YC, et al. Endothelial monocyte-activating polypeptide II: A novel tumor-derived polypeptide that activates host-response mechanisms. J Biol Chem. 1992;267(28):20239–20247. doi: 10.1016/S0021-9258(19)88692-1. - DOI - PubMed
-
- Kao J, Fan YG, Haehnel I, Brett J, Greenberg S, Clauss M, Kayton M, Houck K, Kisiel W, Seljelid R, et al. A peptide derived from the amino terminus of endothelial-monocyte-activating polypeptide II modulates mononuclear and polymorphonuclear leukocyte functions defines an apparently novel cellular interaction site and induces an acute inflammatory response. J Biol Chem. 1994;269(13):9774–9782. doi: 10.1016/S0021-9258(17)36950-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

