The Role of IgE in Upper and Lower Airway Disease: More Than Just Allergy!
- PMID: 34536215
- PMCID: PMC8818003
- DOI: 10.1007/s12016-021-08901-1
The Role of IgE in Upper and Lower Airway Disease: More Than Just Allergy!
Abstract
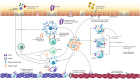
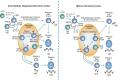
Immunoglobulin E (IgE) is a well-known key factor in allergic airway disease; however, its central role in non-allergic airway inflammation is often underestimated. In some airway diseases, IgE is produced as a result of allergic sensitization. However, in others, IgE production occurs despite the lack of a specific allergen. Although multiple pathways contribute to the production of IgE in airway disease, it is its activity in mediating the inflammatory response that is associated with disease. Therefore, an understanding of IgE as the unifying component of upper and lower airway diseases has important implications for both diagnosis and treatment. Understanding the role of IgE in each upper and lower airway disease highlights its potential utility as a diagnostic marker and therapeutic target. Further classification of these diseases by whether they are IgE mediated or non-IgE mediated, rather than by the existence of an underlying allergic component, accounts for both systemic and localized IgE activity. Improvements in diagnostic methodologies and standardization of clinical practices with this classification in mind can help identify patients with IgE-mediated diseases. In doing so, this group of patients can receive optimal care through targeted anti-IgE therapeutics, which have already demonstrated efficacy across numerous IgE-mediated upper and lower airway diseases.
Keywords: Asthma; Immunoglobulin E; Lower airway disease; Nasal polyps; Rhinitis; Upper airway disease.
© 2021. The Author(s).
Conflict of interest statement
Philippe Gevaert has received consulting fees, honoraria for lectures, and/or research funding from 3NT, Ablynx, ALK, Argenx, AstraZeneca, Bekaert Textiles, Genentech, Inc., Hal Allergy, Medtronic, Novartis, Regeneron, Roche, Sanofi Genzyme, Stallergenes Greer, Teva, and Thermo Fisher. Kit Wong is an employee of Genentech, Inc. Lauren A. Millette is an employee of Genentech, Inc. Tara F. Carr has received consulting fees from Aimmune Therapeutics, AstraZeneca, GlaxoSmithKline, Novartis, Regeneron, and Sanofi Genzyme.
Figures
Similar articles
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
-
Upper and lower airway pathology in young children with allergic- and non-allergic rhinitis.Dan Med Bull. 2011 May;58(5):B4278. Dan Med Bull. 2011. PMID: 21535990 Review.
-
Prevalence of serum IgE antibodies to the Staphylococcus aureus enterotoxins (SAE, SEB, SEC, SED, TSST-1) in patients with persistent allergic rhinitis.Int Arch Allergy Immunol. 2004 Mar;133(3):261-6. doi: 10.1159/000076833. Epub 2004 Feb 17. Int Arch Allergy Immunol. 2004. PMID: 14976395
-
Mucosal IgE immune responses in respiratory diseases.Curr Opin Pharmacol. 2019 Jun;46:100-107. doi: 10.1016/j.coph.2019.05.009. Epub 2019 Jun 17. Curr Opin Pharmacol. 2019. PMID: 31220711 Review.
-
[Diagnosis and consultation rates of allergic diseases in 11-year old schoolchildren, and usefulness of allergy screening test at school].Arerugi. 2012 Jan;61(1):41-50. Arerugi. 2012. PMID: 22398427 Japanese.
Cited by
-
Biologic Therapy in Severe Asthma: A Phenotype-Driven and Targeted Approach.J Clin Med. 2025 Jul 4;14(13):4749. doi: 10.3390/jcm14134749. J Clin Med. 2025. PMID: 40649123 Free PMC article. Review.
-
Endotyping Chronic Respiratory Diseases: T2 Inflammation in the United Airways Model.Life (Basel). 2024 Jul 19;14(7):899. doi: 10.3390/life14070899. Life (Basel). 2024. PMID: 39063652 Free PMC article. Review.
-
Serum T2-High Inflammation Mediators in Eosinophilic COPD.Biomolecules. 2024 Dec 21;14(12):1648. doi: 10.3390/biom14121648. Biomolecules. 2024. PMID: 39766355 Free PMC article.
-
Potential use of plant secondary metabolites in the treatment of allergic respiratory diseases (ARDs).Mol Biol Rep. 2025 Jun 26;52(1):639. doi: 10.1007/s11033-025-10770-2. Mol Biol Rep. 2025. PMID: 40569507 Review.
-
Autoallergy and what the Allergist Needs to know.Curr Allergy Asthma Rep. 2025 Aug 9;25(1):34. doi: 10.1007/s11882-025-01215-8. Curr Allergy Asthma Rep. 2025. PMID: 40782271 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

