Articular and Extra-Articular Benefits in ACR20 Non-responders at Week 104 Treated With Apremilast: Pooled Analysis of Three Randomized Controlled Trials
- PMID: 34536218
- PMCID: PMC8572179
- DOI: 10.1007/s40744-021-00369-x
Articular and Extra-Articular Benefits in ACR20 Non-responders at Week 104 Treated With Apremilast: Pooled Analysis of Three Randomized Controlled Trials
Abstract
Introduction: PALACE 1, 2, and 3 were phase 3 studies aimed to evaluate apremilast efficacy and safety in patients with active psoriatic arthritis (PsA) despite prior treatment with conventional disease-modifying anti-rheumatic drugs and/or biologics. The pooled analysis reported here further characterized the clinical outcomes associated with long-term apremilast exposure in patients failing to achieve ≥ 20% improvement in the American College of Rheumatology response criteria (ACR20) at Week 104.
Methods: Patients randomized to apremilast 30 mg twice daily at baseline and classified as ACR20 non-responders (ACR20NRs) or ACR20 responders (ACR20Rs) at Week 104 were included. Efficacy outcomes included change from baseline to Week 104 in ACR core components and other endpoints.
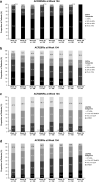
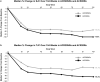
Results: At Week 104, a total of 109 patients were ACR20NRs and 193 were ACR20Rs. As expected, the ACR20R group had improvements in all indices assessed. The ACR20NR group demonstrated substantial mean improvements from baseline in swollen joint count (SJC; - 58%), tender joint count (TJC; - 42%), and Physician's Global Assessment of Disease Activity (PhGA; - 44%); resolution of enthesitis (34%) and dactylitis (68%); and achievement of ≥ 75% reduction from baseline Psoriasis Area and Severity Index scores (among patients with psoriasis involving ≥ 3% of the body surface area) (36%).
Conclusion: Despite not fulfilling a formal ACR20 response at Week 104, ACR20NRs experienced sustained improvements in several PsA core domains, including SJC, TJC, enthesitis, dactylitis, and psoriasis, as well as the PhGA (visual analog scale) scores, with apremilast treatment.
Trial registration: ClinicalTrials.gov identifier: NCT01172938, NCT01212757, and NCT01212770.
Keywords: ACR20 non-responders; Apremilast; Articular; Extra-articular; Psoriatic arthritis.
© 2021. The Author(s).
Figures

References
-
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3) Ann Rheum Dis. 2016;75(6):1065–1073. doi: 10.1136/annrheumdis-2015-207963. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

