The CNS-penetrant soluble guanylate cyclase stimulator CYR119 attenuates markers of inflammation in the central nervous system
- PMID: 34537066
- PMCID: PMC8449877
- DOI: 10.1186/s12974-021-02275-z
The CNS-penetrant soluble guanylate cyclase stimulator CYR119 attenuates markers of inflammation in the central nervous system
Abstract
Background: Inflammation in the central nervous system (CNS) is observed in many neurological disorders. Nitric oxide-soluble guanylate cyclase-cyclic guanosine monophosphate (NO-sGC-cGMP) signaling plays an essential role in modulating neuroinflammation. CYR119 is a CNS-penetrant sGC stimulator that amplifies endogenous NO-sGC-cGMP signaling. We evaluated target engagement and the effects of CYR119 on markers of neuroinflammation in vitro in mouse microglial cells and in vivo in quinolinic acid (QA)-induced and high-fat diet-induced rodent neuroinflammation models.
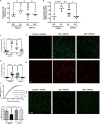
Methods: Target engagement was verified in human embryonic kidney (HEK) cells, rat primary neurons, mouse SIM-A9 cells, and in rats by measuring changes in cGMP and downstream targets of sGC signaling [phosphorylated vasodilator-stimulated phosphoprotein (pVASP), phosphorylated cAMP-response element binding (pCREB)]. In SIM-A9 cells stimulated with lipopolysaccharides (LPS), markers of inflammation were measured when cells were treated with or without CYR119. In rats, microinjections of QA and vehicle were administered into the right and left hemispheres of striatum, respectively, and then rats were dosed daily with either CYR119 (10 mg/kg) or vehicle for 7 days. The activation of microglia [ionized calcium binding adaptor molecule 1 (Iba1)] and astrocytes [glial fibrillary acidic protein (GFAP)] was measured by immunohistochemistry. Diet-induced obese (DIO) mice were treated daily with CYR119 (10 mg/kg) for 6 weeks, after which inflammatory genetic markers were analyzed in the prefrontal cortex.
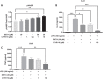
Results: In vitro, CYR119 synergized with exogenous NO to increase the production of cGMP in HEK cells and in primary rat neuronal cell cultures. In primary neurons, CYR119 stimulated sGC, resulting in accumulation of cGMP and phosphorylation of CREB, likely through the activation of protein kinase G (PKG). CYR119 attenuated LPS-induced elevation of interleukin 6 (IL-6) and tumor necrosis factor (TNF) in mouse microglial cells. Following oral dosing in rats, CYR119 crossed the blood-brain barrier (BBB) and stimulated an increase in cGMP levels in the cerebral spinal fluid (CSF). In addition, levels of proinflammatory markers associated with QA administration or high-fat diet feeding were lower in rodents treated with CYR119 than in those treated with vehicle.
Conclusions: These data suggest that sGC stimulation could provide neuroprotective effects by attenuating inflammatory responses in nonclinical models of neuroinflammation.
Keywords: CREB; High-fat diet; Microglia; Neuroinflammation; Nitric oxide; Quinolinic acid; Soluble guanylate cyclase; cGMP; sGC.
© 2021. The Author(s).
Conflict of interest statement
The authors are/were employed by Ironwood Pharmaceuticals and Cyclerion Therapeutics and may hold stock in Cyclerion Therapeutics.
Figures

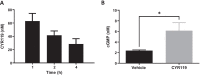

Similar articles
-
Contribution of iNOS/sGC/PKG pathway, COX-2, CYP4A1, and gp91(phox) to the protective effect of 5,14-HEDGE, a 20-HETE mimetic, against vasodilation, hypotension, tachycardia, and inflammation in a rat model of septic shock.Nitric Oxide. 2013 Sep 1;33:18-41. doi: 10.1016/j.niox.2013.05.001. Epub 2013 May 14. Nitric Oxide. 2013. PMID: 23684565 Free PMC article.
-
Pharmacological Characterization of IW-1973, a Novel Soluble Guanylate Cyclase Stimulator with Extensive Tissue Distribution, Antihypertensive, Anti-Inflammatory, and Antifibrotic Effects in Preclinical Models of Disease.J Pharmacol Exp Ther. 2018 Jun;365(3):664-675. doi: 10.1124/jpet.117.247429. Epub 2018 Apr 11. J Pharmacol Exp Ther. 2018. PMID: 29643251
-
Nitric oxide-independent down-regulation of soluble guanylyl cyclase by bacterial endotoxin in astroglial cells.J Neurochem. 1999 Nov;73(5):2149-57. J Neurochem. 1999. PMID: 10537075
-
The nitric oxide-soluble guanylate cyclase-cGMP pathway in pulmonary hypertension: from PDE5 to soluble guanylate cyclase.Eur Respir Rev. 2024 Mar 20;33(171):230183. doi: 10.1183/16000617.0183-2023. Print 2024 Jan 31. Eur Respir Rev. 2024. PMID: 38508664 Free PMC article. Review.
-
Effects of the NO/soluble guanylate cyclase/cGMP system on the functions of human platelets.Nitric Oxide. 2018 Jun 1;76:71-80. doi: 10.1016/j.niox.2018.03.008. Epub 2018 Mar 14. Nitric Oxide. 2018. PMID: 29550521 Review.
Cited by
-
Emerging Roles of Endothelial Nitric Oxide in Preservation of Cognitive Health.Stroke. 2023 Mar;54(3):686-696. doi: 10.1161/STROKEAHA.122.041444. Epub 2023 Feb 27. Stroke. 2023. PMID: 36848426 Free PMC article. Review.
-
Neuroprotective Effect of a Novel Soluble Guanylate Cyclase Activator Runcaciguat in Diabetic and Ischemic Retinopathy.Diabetes. 2025 Jul 1;74(7):1220-1232. doi: 10.2337/db24-0739. Diabetes. 2025. PMID: 40249725
-
First-in-human trial to assess safety, tolerability, pharmacokinetics, and pharmacodynamics of zagociguat (CY6463), a CNS-penetrant soluble guanylyl cyclase stimulator.Clin Transl Sci. 2023 Aug;16(8):1381-1395. doi: 10.1111/cts.13537. Epub 2023 May 3. Clin Transl Sci. 2023. PMID: 37118895 Free PMC article. Clinical Trial.
-
Targeting resident astrocytes attenuates neuropathic pain after spinal cord injury.Elife. 2024 Nov 15;13:RP95672. doi: 10.7554/eLife.95672. Elife. 2024. PMID: 39545839 Free PMC article.
-
Multifaceted role of nitric oxide in vascular dementia.Med Gas Res. 2025 Dec 1;15(4):496-506. doi: 10.4103/mgr.MEDGASRES-D-24-00158. Epub 2025 Apr 29. Med Gas Res. 2025. PMID: 40300885 Free PMC article. Review.
References
-
- Tobin JV, Zimmer DP, Shea C, Germano P, Bernier SG, Liu G, et al. Pharmacological characterization of IW-1973, a novel soluble guanylate cyclase stimulator with extensive tissue distribution, antihypertensive, anti-inflammatory, and antifibrotic effects in preclinical models of disease. J Pharmacol Exp Ther. 2018;365(3):664–675. doi: 10.1124/jpet.117.247429. - DOI - PubMed
-
- Zimmer DP, Shea CM, Tobin JV, Tchernychev B, Germano P, Sykes K, et al. Olinciguat, an Oral sGC stimulator, exhibits diverse pharmacology across preclinical models of cardiovascular, metabolic, renal, and inflammatory disease. Front Pharmacol. 2020;11:419. doi: 10.3389/fphar.2020.00419. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

