Impact of cobas PCR Media freezing on SARS-CoV-2 viral RNA integrity and whole genome sequencing analyses
- PMID: 34537474
- PMCID: PMC8379003
- DOI: 10.1016/j.diagmicrobio.2021.115521
Impact of cobas PCR Media freezing on SARS-CoV-2 viral RNA integrity and whole genome sequencing analyses
Abstract
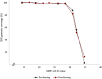
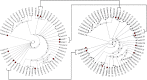
SARS-CoV-2 whole genome sequencing is a molecular biology tool performed to support many aspects of the response to the pandemic. Freezing of primary clinical nasopharyngeal swabs and shipment to reference laboratories is usually required for sequencing. Cobas PCR Media transport medium facilitates high throughput SARS-CoV-2 RT-PCR analyses on cobas platforms. The manufacturer doesn't recommend freezing this transport medium because of risks of degrading molecular templates and impairing test results. Our objective was to compare the quality and results of SARS-CoV-2 genomic sequencing when performed on fresh or frozen samples in cobas PCR Media. Viral genome sequencing was performed using Oxford Nanopore Technologies MinION platform. Sequencing performance, quality and results did not significantly differ between fresh and frozen samples (n = 10). Freezing of cobas PCR Media does not negatively affect SARS-CoV-2 RNA sequencing results and it is therefore a suitable transport medium for outsourcing sequencing analyses to reference laboratories.
Keywords: Cobas PCR Media; Molecular biology; SARS-CoV-2; Transport Medium; Whole Genome Sequencing.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures

References
-
- Boutin CA, Grandjean-Lapierre S, Gagnon S, Labbe AC, Charest H, Roger M, et al. Comparison of SARS-CoV-2 detection from combined nasopharyngeal/oropharyngeal swab samples by a laboratory-developed real-time RT-PCR test and the Roche SARS-CoV-2 assay on a cobas 8800 instrument. J Clin Virol. 2020;132 - PMC - PubMed
-
- GISAID . 2020. Genomic epidemiology of hCoV-19.https://www.gisaid.org/ Accessed December 22, 2020.
-
- Khiri H, Camus C, Portugal M, Penaranda G, Boyer S, Halfon P. Cytological and virological medium performance and stability assessment using the Cobas 4800 HPV test (Roche Diagnostics) used in France. Ann Biol Clin. 2014;72:213–223. Paris. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

