Ca2+ transients in ICC-MY define the basis for the dominance of the corpus in gastric pacemaking
- PMID: 34537580
- PMCID: PMC8592010
- DOI: 10.1016/j.ceca.2021.102472
Ca2+ transients in ICC-MY define the basis for the dominance of the corpus in gastric pacemaking
Abstract
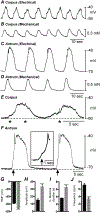
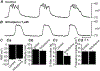
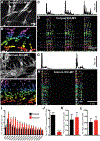
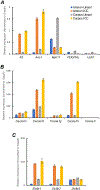
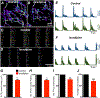
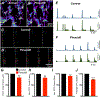
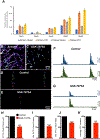
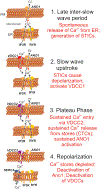
Myenteric interstitial cells of Cajal (ICC-MY) generate and actively propagate electrical slow waves in the stomach. Slow wave generation and propagation are altered in gastric motor disorders, such as gastroparesis, and the mechanism for the gradient in slow wave frequency that facilitates proximal to distal propagation of slow waves and normal gastric peristalsis is poorly understood. Slow waves depend upon Ca2+-activated Cl- channels (encoded by Ano1). We characterized Ca2+ signaling in ICC-MY in situ using mice engineered to have cell-specific expression of GCaMP6f in ICC. Ca2+ signaling differed in ICC-MY in corpus and antrum. Localized Ca2+ transients were generated from multiple firing sites and were organized into Ca2+ transient clusters (CTCs). Ca2+ transient refractory periods occurred upon cessation of CTCs, but a relatively higher frequency of Ca2+ transients persisted during the inter-CTC interval in corpus than in antrum ICC-MY. The onset of Ca2+ transients after the refractory period was associated with initiation of the next CTC. Thus, CTCs were initiated at higher frequencies in corpus than in antrum ICC-MY. Initiation and propagation of CTCs (and electrical slow waves) depends upon T-type Ca2+ channels, and durations of CTCs relied upon L-type Ca2+ channels. The durations of CTCs mirrored the durations of slow waves. CTCs and Ca2+ transients between CTCs resulted from release of Ca2+ from intracellular stores and were maintained, in part, by store-operated Ca2+ entry. Our data suggest that Ca2+ release and activation of Ano1 channels both initiate and contribute to the plateau phase of slow waves.
Keywords: Gastric motility; Interstitial cells; Pacemaker activity; Slow waves.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration Competing Interest
Authors declare that they have no competing interests
Figures







References
-
- Tack J, Gastric motor and sensory function, Curr Opin Gastroenterol 21 (2005) 665–672. - PubMed
-
- van den Elzen BD, Boeckxstaens GE, Review article: a critical view on impaired accommodation as therapeutic target for functional dyspepsia, Aliment Pharmacol Ther 23 (2006) 1499–1510. - PubMed
-
- Kelly KA, Code CF, Elveback LR, Patterns of canine gastric electrical activity, Am J Physiol 217 (1969) 461–470. - PubMed
-
- Szurszewski JH, Electrical basis for gastrointestinal motility, in: Johnson LR (Ed.), Physiology of the Gastrointestinal Tract, Raven Press, New York, 1987, pp. 383–422.
-
- Ozaki H, Stevens RJ, Blondfield DP, Publicover NG, Sanders KM, Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles, Am J Physiol 260 (1991) C917–C925. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

