Safety and Immunogenicity of an Inactivated Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine in a Subgroup of Healthy Adults in Chile
- PMID: 34537835
- PMCID: PMC9402626
- DOI: 10.1093/cid/ciab823
Safety and Immunogenicity of an Inactivated Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine in a Subgroup of Healthy Adults in Chile
Abstract
Background: The development of effective vaccines against coronavirus disease 2019 is a global priority. CoronaVac is an inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine with promising safety and immunogenicity profiles. This article reports safety and immunogenicity results obtained for healthy Chilean adults aged ≥18 years in a phase 3 clinical trial.
Methods: Volunteers randomly received 2 doses of CoronaVac or placebo, separated by 2 weeks. A total of 434 volunteers were enrolled, 397 aged 18-59 years and 37 aged ≥60 years. Solicited and unsolicited adverse reactions were registered from all volunteers. Blood samples were obtained from a subset of volunteers and analyzed for humoral and cellular measures of immunogenicity.
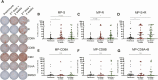
Results: The primary adverse reaction in the 434 volunteers was pain at the injection site, with a higher incidence in the vaccine than in the placebo arm. Adverse reactions observed were mostly mild and local. No severe adverse events were reported. The humoral evaluation was performed on 81 volunteers. Seroconversion rates for specific anti-S1-receptor binding domain (RBD) immunoglobulin G (IgG) were 82.22% and 84.44% in the 18-59 year age group and 62.69% and 70.37% in the ≥60 year age group, 2 and 4 weeks after the second dose, respectively. A significant increase in circulating neutralizing antibodies was detected 2 and 4 weeks after the second dose. The cellular evaluation was performed on 47 volunteers. We detected a significant induction of T-cell responses characterized by the secretion of interferon-γ (IFN-γ) upon stimulation with Mega Pools of peptides from SARS-CoV-2.
Conclusions: Immunization with CoronaVac in a 0-14 schedule in Chilean adults aged ≥18 years is safe, induces anti-S1-RBD IgG with neutralizing capacity, activates T cells, and promotes the secretion of IFN-γ upon stimulation with SARS-CoV-2 antigens.
Keywords: COVID-19; CoronaVac; SARS-CoV-2; phase 3 clinical trial; vaccines.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures



Update of
-
Interim report: Safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 in healthy chilean adults in a phase 3 clinical trial.medRxiv [Preprint]. 2021 Apr 1:2021.03.31.21254494. doi: 10.1101/2021.03.31.21254494. medRxiv. 2021. Update in: Clin Infect Dis. 2022 Aug 24;75(1):e792-e804. doi: 10.1093/cid/ciab823. PMID: 35441164 Free PMC article. Updated. Preprint.
References
-
- Zhu N, Zhang D, Wang W, et al. . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–733. Available at: http://www.ncbi.nlm.nih.gov/pubmed/31978945. - PMC - PubMed
-
- Kim D, Lee J-Y, Yang J-S, Kim JW, Kim VN, Chang H. The architecture of SARS-CoV-2 transcriptome. Cell 2020; 181:914–21.e10. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32330414. - PMC - PubMed
-
- Zhou P, Yang X-L, Wang X-G, et al. . A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32015507. - PMC - PubMed
-
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20:533–4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32087114. - PMC - PubMed
-
- Perez-Saez J, Lauer SA, Kaiser L, et al. . Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect Dis 2020; Available at: http://www.ncbi.nlm.nih.gov/pubmed/32679085. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

