Impact of Body Mass Index on the Efficacy of Biological Therapies in Patients with Psoriasis: A Real-World Study
- PMID: 34537921
- PMCID: PMC8481196
- DOI: 10.1007/s40261-021-01080-z
Impact of Body Mass Index on the Efficacy of Biological Therapies in Patients with Psoriasis: A Real-World Study
Abstract
Background: The efficacy of biological therapies used for the treatment of chronic plaque psoriasis can be influenced by numerous variables including body mass index (BMI).
Objective: This study aimed to evaluate the impact of BMI on the short-term and long-term efficacy of biological therapies in clinical practice and to identify the best therapeutic options in obese patients (BMI ≥ 30 kg/m2).
Methods: A multicentric retrospective study was conducted in patients who initiated a biological therapy during the period January 2006-December 2019. The proportion of patients achieving a 90% improvement of baseline Psoriasis Area and Severity Index at weeks 12 and 24 was calculated also recording the 12- and 24-month drug survival as a measure of long-term efficacy, performing multivariate analyses to assess the impact of different variables.
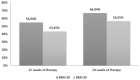
Results: Five hundred and four patients with psoriasis were included. After 12 and 24 weeks, the proportion of patients achieving a 90% improvement of baseline Psoriasis Area and Severity Index response was higher in patients with a BMI < 30 kg/m2 compared with those with a BMI ≥ 30 kg/m2 [54.90% vs 43.45% (p = 0.014) at week 12 and 66.84% vs 56.55% (p = 0.021) at week 24]. The Kaplan-Meier survival curves showed how obese patients had a higher probability of discontinuation due to a lack or loss of efficacy (p = 0.0192) compared with non-obese patients. The drug survival analysis also showed that BMI negatively affected the drug survival of secukinumab (odds ratio 1.27, p < 0.001) and ustekinumab (odds ratio 1.06, p = 0.050), while the long-term efficacy of adalimumab, etanercept, and ixekizumab was not influenced by BMI.
Conclusions: Obesity (BMI ≥ 30 kg/m2) negatively affects the clinical response of biological drugs in psoriatic patients, with anti-interleukin drugs being more affected by BMI than anti-tumor necrosis factor drugs.
© 2021. The Author(s).
Conflict of interest statement
Giacomo Caldarola reports consulting fees or honorarium and payment for lectures from Lilly and Novartis, outside the submitted work. Andrea Chiricozzi reports consulting fees or honorarium from Abbvie, Novartis, Lilly, Leo Pharma, Janssen, Sanofi, UCB Pharma, and Pfizer, outside the submitted work. Clara De Simone reports consulting fees or honorarium from Abbvie, Amgen, Novartis, Celgene, Sanofi, UCB Pharma, Janssen, and Lilly and payment for lectures from Abbvie, Lilly, Novartis, UCB Pharma, and Celgene, outside the submitted work. Ketty Peris reports consulting fees or honorarium from Almirall, AbbVie, Biogen, Lilly, Celgene, Galderma, Leo Pharma, Novartis, Pierre Fabre, Sanofi, Sandoz, Sun Pharma, and Janssen, outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

References
-
- Prignano F, Rogai V, Cavallucci E, et al. Epidemiology of psoriasis and psoriatic arthritis in Italy-a systematic review. Curr Rheumatol Rep. 2018;20(7):43. - PubMed
-
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Investig Dermatol. 2009;129(6):1339–1350. - PubMed
-
- Amin M, No DJ, Egeberg A, Wu JJ. Choosing first-line biologic treatment for moderate-to-severe psoriasis: what does the evidence say? Am J Clin Dermatol. 2018;19(1):1–13. - PubMed
-
- Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl.):s176–s185. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

