Roxadustat for the Maintenance Treatment of Anemia in Patients with End-Stage Kidney Disease on Stable Dialysis: A European Phase 3, Randomized, Open-Label, Active-Controlled Study (PYRENEES)
- PMID: 34537926
- PMCID: PMC8478768
- DOI: 10.1007/s12325-021-01904-6
Roxadustat for the Maintenance Treatment of Anemia in Patients with End-Stage Kidney Disease on Stable Dialysis: A European Phase 3, Randomized, Open-Label, Active-Controlled Study (PYRENEES)
Abstract
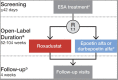
Introduction: Roxadustat is an orally administered hypoxia-inducible factor prolyl hydroxylase inhibitor being developed for the treatment of anemia of chronic kidney disease (CKD). This European, phase 3, randomized, open-label, active-controlled study investigated efficacy and safety of roxadustat in patients with end-stage kidney disease on dialysis for at least 4 months.
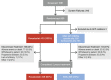
Methods: Patients were randomized to switch from an erythropoiesis-stimulating agent (ESA) (epoetin alfa or darbepoetin alfa) to roxadustat three times/week or to continue their previous ESA. Roxadustat and ESA doses were adjusted to maintain hemoglobin within 10.0-12.0 g/dL during the treatment period (day 1 up to 52-104 weeks). Primary endpoints were hemoglobin change from baseline (CFB) to the average of weeks 28-36 without rescue therapy and hemoglobin CFB to the average of weeks 28-52 regardless of rescue therapy. Treatment-emergent adverse events (TEAEs) were assessed descriptively.
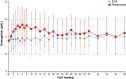
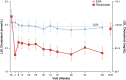
Results: Of 1081 screened patients, 836 were randomized and received treatment (roxadustat, n = 415; ESA, n = 421). The least squares means (95% CI) of the treatment difference (roxadustat - ESA) for hemoglobin CFB to weeks 28-36 (without rescue therapy) and CFB to weeks 28-52 (regardless of rescue therapy) were 0.235 (0.132, 0.339) g/dL and 0.171 (0.082, 0.261) g/dL, respectively, demonstrating non-inferiority of roxadustat to ESA (non-inferiority margin of - 0.75 g/dL). The proportions of patients who achieved target hemoglobin without rescue therapy during weeks 28-36 were 84.2% (roxadustat) and 82.4% (ESA). Roxadustat was superior to ESA in decreasing LDL cholesterol from baseline to the average of weeks 12-28. Serious TEAEs occurred in 50.7% (roxadustat) and 45.0% (ESA) of patients. Common TEAEs in both treatment groups included hypertension, arteriovenous fistula thrombosis, headache, and diarrhea.
Conclusion: Roxadustat was non-inferior to ESAs in maintaining hemoglobin levels in this cohort of patients with anemia of CKD on dialysis for at least 4 months who were previously treated with ESAs. Observed TEAEs were consistent with previous studies.
Keywords: Chronic kidney disease; Hemodialysis; Peritoneal dialysis.
© 2021. The Author(s).
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

