Inhibition of WEE1 Is Effective in TP53- and RAS-Mutant Metastatic Colorectal Cancer: A Randomized Trial (FOCUS4-C) Comparing Adavosertib (AZD1775) With Active Monitoring
- PMID: 34538072
- PMCID: PMC8601321
- DOI: 10.1200/JCO.21.01435
Inhibition of WEE1 Is Effective in TP53- and RAS-Mutant Metastatic Colorectal Cancer: A Randomized Trial (FOCUS4-C) Comparing Adavosertib (AZD1775) With Active Monitoring
Abstract
Purpose: Outcomes in RAS-mutant metastatic colorectal cancer (mCRC) remain poor and patients have limited therapeutic options. Adavosertib is the first small-molecule inhibitor of WEE1 kinase. We hypothesized that aberrations in DNA replication seen in mCRC with both RAS and TP53 mutations would sensitize tumors to WEE1 inhibition.
Methods: Patients with newly diagnosed mCRC were registered into FOCUS4 and tested for TP53 and RAS mutations. Those with both mutations who were stable or responding after 16 weeks of chemotherapy were randomly assigned 2:1 between adavosertib and active monitoring (AM). Adavosertib (250 mg or 300 mg) was taken orally once on days 1-5 and days 8-12 of a 3-week cycle. The primary outcome was progression-free survival (PFS), with a target hazard ratio (HR) of 0.5 and 80% power with a one-sided 0.025 significance level.
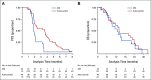
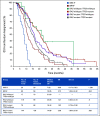
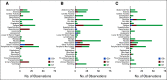
Results: FOCUS4-C was conducted between April 2017 and Mar 2020 during which time 718 patients were registered; 247 (34%) were RAS/TP53-mutant. Sixty-nine patients were randomly assigned from 25 UK hospitals (adavosertib = 44; AM = 25). Adavosertib was associated with a PFS improvement over AM (median 3.61 v 1.87 months; HR = 0.35; 95% CI, 0.18 to 0.68; P = .0022). Overall survival (OS) was not improved with adavosertib versus AM (median 14.0 v 12.8 months; HR = 0.92; 95% CI, 0.44 to 1.94; P = .93). In prespecified subgroup analysis, adavosertib activity was greater in left-sided tumors (HR = 0.24; 95% CI, 0.11 to 0.51), versus right-sided (HR = 1.02; 95% CI, 0.41 to 2.56; interaction P = .043). Adavosertib was well-tolerated; grade 3 toxicities were diarrhea (9%), nausea (5%), and neutropenia (7%).
Conclusion: In this phase II randomized trial, adavosertib improved PFS compared with AM and demonstrates potential as a well-tolerated therapy for RAS/TP53-mutant mCRC. Further testing is required in this sizable population of unmet need.
Conflict of interest statement
Figures


References
-
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. - PubMed
-
- Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. - PubMed
-
- Aarts M, Sharpe R, Garcia-Murillas I, et al. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discov. 2012;2:524–539. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

