Anesthesia can alter the levels of corticosterone and the phosphorylation of signaling molecules
- PMID: 34538274
- PMCID: PMC8451088
- DOI: 10.1186/s13104-021-05763-w
Anesthesia can alter the levels of corticosterone and the phosphorylation of signaling molecules
Abstract
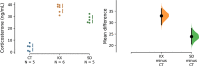
Objective: Neuroscience research using laboratory animals has increased over the years for a number of reasons. Some of these studies require the use of anesthetics for surgical procedures. However, the use of anesthetics promotes several physiological changes that may interfere with experimental results. Although the anesthetics and methods of delivery used to vary, one of the most common is ketamine associated with another compound such as xylazine. We aimed to evaluate the effect of ketamine and xylazine (KX) on corticosterone levels and on the degree of phosphorylation of p44/42 (ERK1/2), Src kinases and calcium/calmodulin-dependent kinase II (CAMKII). We also compared the effects of KX on sleep deprivation, which is known to affect the hormonal profile including corticosterone.
Results: We found that the use of KX can increase corticosterone levels and alter the degree of phosphorylation of signaling proteins.
Keywords: Corticosterone; Ketamine; Signaling molecules; Sleep deprivation; Xylazine.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Flecknell P. Replacement, reduction and refinement. Altex. 2002;19(2):73–78. - PubMed
MeSH terms
Substances
Grants and funding
- 2011/15962-7/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2013/00152-5/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2016/04297-6/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2017/10404-2/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 467566/2014-3/Conselho Nacional de Desenvolvimento Científico e Tecnológico
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

