RAMPART: A phase III multi-arm multi-stage trial of adjuvant checkpoint inhibitors in patients with resected primary renal cell carcinoma (RCC) at high or intermediate risk of relapse
- PMID: 34538402
- PMCID: PMC8520913
- DOI: 10.1016/j.cct.2021.106482
RAMPART: A phase III multi-arm multi-stage trial of adjuvant checkpoint inhibitors in patients with resected primary renal cell carcinoma (RCC) at high or intermediate risk of relapse
Abstract
Background: 20-60% of patients with initially locally advanced Renal Cell Carcinoma (RCC) develop metastatic disease despite optimal surgical excision. Adjuvant strategies have been tested in RCC including cytokines, radiotherapy, hormones and oral tyrosine-kinase inhibitors (TKIs), with limited success. The predominant global standard-of-care after nephrectomy remains active monitoring. Immune checkpoint inhibitors (ICIs) are effective in the treatment of metastatic RCC; RAMPART will investigate these agents in the adjuvant setting.
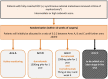
Methods/design: RAMPART is an international, UK-led trial investigating the addition of ICIs after nephrectomy in patients with resected locally advanced RCC. RAMPART is a multi-arm multi-stage (MAMS) platform trial, upon which additional research questions may be addressed over time. The target population is patients with histologically proven resected locally advanced RCC (clear cell and non-clear cell histological subtypes), with no residual macroscopic disease, who are at high or intermediate risk of relapse (Leibovich score 3-11). Patients with fully resected synchronous ipsilateral adrenal metastases are included. Participants are randomly assigned (3,2:2) to Arm A - active monitoring (no placebo) for one year, Arm B - durvalumab (PD-L1 inhibitor) 4-weekly for one year; or Arm C - combination therapy with durvalumab 4-weekly for one year plus two doses of tremelimumab (CTLA-4 inhibitor) at day 1 of the first two 4-weekly cycles. The co-primary outcomes are disease-free-survival (DFS) and overall survival (OS). Secondary outcomes include safety, metastasis-free survival, RCC specific survival, quality of life, and patient and clinician preferences. Tumour tissue, plasma and urine are collected for molecular analysis (TransRAMPART).
Trial registration: ISRCTN #: ISRCTN53348826, NCT #: NCT03288532, EUDRACT #: 2017-002329-39, CTA #: 20363/0380/001-0001, MREC #: 17/LO/1875, ClinicalTrials.gov Identifier: NCT03288532, RAMPART grant number: MC_UU_12023/25, TransRAMPART grant number: A28690 Cancer Research UK, RAMPART Protocol version 5.0.
Keywords: Check-point inhibitor; Durvalumab; MAMS; Platform trial; RAMPART; Renal cancer; Tremelimumab.
Crown Copyright © 2021. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
- Dabestani S. Long-term outcomes of follow-up for initially localised clear cell renal cell carcinoma: RECUR database analysis. Eur. Urol. Focus. 2019;5(5):857–866. - PubMed
-
- Dabestani S. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J. Urol. 2016;34(8):1081–1086. - PubMed
-
- Lam J.S. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J. Urol. 2005;174(2):466–472. discussion 472. (quiz 801) - PubMed
-
- Weight C.J. The role of Adrenalectomy in renal Cancer. Eur. Urol. Focus. 2016;1(3):251–257. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials