Thymus Extracellular Matrix-Derived Scaffolds Support Graft-Resident Thymopoiesis and Long-Term In Vitro Culture of Adult Thymic Epithelial Cells
- PMID: 34539304
- PMCID: PMC8436951
- DOI: 10.1002/adfm.202010747
Thymus Extracellular Matrix-Derived Scaffolds Support Graft-Resident Thymopoiesis and Long-Term In Vitro Culture of Adult Thymic Epithelial Cells
Abstract
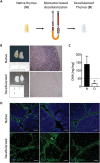
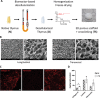
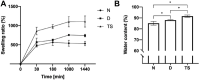
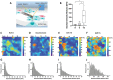
The thymus provides the physiological microenvironment critical for the development of T lymphocytes, the cells that orchestrate the adaptive immune system to generate an antigen-specific response. A diverse population of stroma cells provides surface-bound and soluble molecules that orchestrate the intrathymic maturation and selection of developing T cells. Forming an intricate 3D architecture, thymic epithelial cells (TEC) represent the most abundant and important constituent of the thymic stroma. Effective models for in and ex vivo use of adult TEC are still wanting, limiting the engineering of functional thymic organoids and the understanding of the development of a competent immune system. Here a 3D scaffold is developed based on decellularized thymic tissue capable of supporting in vitro and in vivo thymopoiesis by both fetal and adult TEC. For the first time, direct evidences of feasibility for sustained graft-resident T-cell development using adult TEC as input are provided. Moreover, the scaffold supports prolonged in vitro culture of adult TEC, with a retained expression of the master regulator Foxn1. The success of engineering a thymic scaffold that sustains adult TEC function provides unprecedented opportunities to investigate thymus development and physiology and to design and implement novel strategies for thymus replacement therapies.
Keywords: 3D scaffolds; decellularization; thymic epithelial cells; thymus engineering.
© 2021 The Authors. Advanced Functional Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
Philipp Oertle is a shareholder in ARTIDIS AG. Gitika Srivastava and Philipp Oertle are employed by ARTIDIS AG. The other authors declare no conflict of interest.
Figures





References
-
- Miller J. F. A. P., Nat. Rev. Immunol. 2011, 11, 489. - PubMed
-
- Abramson J., Anderson G., Annu. Rev. Immunol. 2017, 35, 85. - PubMed
-
- Kadouri N., Nevo S., Goldfarb Y., Abramson J., Nat. Rev. Immunol. 2019, 20, 239. - PubMed
-
- Takahama Y., Ohigashi I., Baik S., Anderson G., Nat. Rev. Immunol. 2017, 17, 295. - PubMed
LinkOut - more resources
Full Text Sources
