Serum Soluble Tumor Necrosis Factor Receptors 1 and 2 Are Early Prognosis Markers After ST-Segment Elevation Myocardial Infarction
- PMID: 34539391
- PMCID: PMC8440863
- DOI: 10.3389/fphar.2021.656928
Serum Soluble Tumor Necrosis Factor Receptors 1 and 2 Are Early Prognosis Markers After ST-Segment Elevation Myocardial Infarction
Abstract
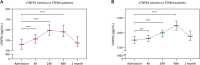
Background: As inflammation following ST-segment elevation myocardial infarction (STEMI) is both beneficial and deleterious, there is a need to find new biomarkers of STEMI severity. Objective: We hypothesized that the circulating concentration of the soluble tumor necrosis factor α receptors 1 and 2 (sTNFR1 and sTNFR2) might predict clinical outcomes in STEMI patients. Methods: We enrolled into a prospective cohort 251 consecutive STEMI patients referred to our hospital for percutaneous coronary intervention revascularization. Blood samples were collected at five time points: admission and 4, 24, 48 h, and 1 month after admission to assess sTNFR1 and sTNFR2 serum concentrations. Patients underwent cardiac magnetic resonance imaging at 1 month. Results: sTNFR1 concentration increased at 24 h with a median of 580.5 pg/ml [95% confidence interval (CI): 534.4-645.6]. sTNFR2 increased at 48 h with a median of 2,244.0 pg/ml [95% CI: 2090.0-2,399.0]. Both sTNFR1 and sTNFR2 peak levels were correlated with infarct size and left ventricular end-diastolic volume and inversely correlated with left ventricular ejection fraction. Patients with sTNFR1 or sTNFR2 concentration above the median value were more likely to experience an adverse clinical event within 24 months after STEMI [hazards ratio (HR): 8.8, 95% CI: 4.2-18.6, p < 0.0001 for sTNFR1; HR: 6.1, 95% CI: 2.5 -10.5, p = 0.0003 for sTNFR2]. Soluble TNFR1 was an independent predictor of major adverse cardiovascular events and was more powerful than troponin I (p = 0.04 as compared to the troponin AUC). Conclusion: The circulating sTNFR1 and sTNFR2 are inflammatory markers of morphological and functional injury after STEMI. sTNFR1 appears as an early independent predictor of clinical outcomes in STEMI patients.
Keywords: TNF; biomarker; myocardial infarction; prognosis; soluble TNF receptor.
Copyright © 2021 Paccalet, Crola Da Silva, Mechtouff, Amaz, Varillon, de Bourguignon, Cartier, Prieur, Tomasevic, Genot, Leboube, Derimay, Rioufol, Bonnefoy-Cudraz, Mewton, Ovize, Bidaux and Bochaton.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Chung E. S., Packer M., Lo K. H., Fasanmade A. A., Willerson J. T. (2003). Randomized, Double-Blind, Placebo-Controlled, Pilot Trial of Infliximab, a Chimeric Monoclonal Antibody to Tumor Necrosis Factor-α, in Patients with Moderate-To-Severe Heart Failure. Circulation 107, 3133–3140. 10.1161/01.CIR.0000077913.60364.D2 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

