Comparison of Improvement in 2-Year Survival Rate of Patients with Stage II-III Non-Small Cell Lung Cancer Treated with Different Durations of Chinese Patent Medicine: A Retrospective Cohort Study
- PMID: 34539404
- PMCID: PMC8443780
- DOI: 10.3389/fphar.2021.719802
Comparison of Improvement in 2-Year Survival Rate of Patients with Stage II-III Non-Small Cell Lung Cancer Treated with Different Durations of Chinese Patent Medicine: A Retrospective Cohort Study
Abstract
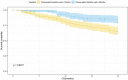
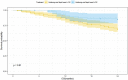
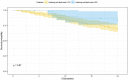
Background: Chinese patent medicine is widely used among patients with malignant tumors, and current studies have shown that long-term treatment with Chinese patent medicine is related to improved outcomes of patients. Huisheng Oral Liquid is a kind of Chinese patent medicine with the effects of curing dispersion-thirst and dissipating blood stasis. However, little is known about how it affects the survival rate of patients. Thus, patients with stage II-III NSCLC (non-small-cell lung cancer) were chosen to participate in a retrospective cohort study, which was conducted to preliminarily investigate the effects of using Chinese patent medicine and Huisheng Oral Liquid for different treatment durations on patients' 2-year survival rate and explore the prognostic factors affecting the 2-year survival rate of those patients. Purpose: This work compares the effect of different durations of treatment with Chinese patent medicine and Huisheng Oral Liquid on the 2-year survival rate of patients with stage II-III NSCLC and explores the prognostic factors of the patients' 2-year survival rate. Methods: This retrospective cohort study included patients with non-small cell lung cancer stage II-III according to the 2015 NCCN Guidelines: Non-Small Cell Lung Cancer. The Kaplan-Meier method was used to compare the 2-year survival rate of patients treated with different durations of Chinese medicine and Huisheng Oral Liquid. The relationship between different treatment durations and degree of improvement of 2-year survival rate was explored using the Cochran-Armitage trend test. The Cox proportional-hazards regression models were used to explore factors affecting the 2-year survival rate of patients. Results: A total of 614 patients with stage II-III NSCLC diagnosed from January 2015 to December 2018 were included in this study. Patients treated with Chinese patent medicine were divided into three groups by treatment durations: < 3 months, ≥ 3 months, and ≥6 months, and those treated with Huisheng Oral Liquid were divided into < 3 months and ≥3 and ≥6 months. The results showed that ① the 2-year survival rate of patients treated with Chinese patent medicine for ≥3 months and ≥6 months was higher than that of patients treated for <3 months and the difference was statistically significant (p < 0.05). Further analysis of Huisheng Oral Liquid treatment revealed that ② the 2-year survival rate of patients treated with Huisheng Oral Liquid for ≥3 months was higher than that of patients treated for <3 months (p < 0.05). Because the total number of patients treated with Huisheng Oral Liquid for ≥6 months and the number of patients with improved outcomes were too small, there was no statistically significant difference in the 2-year survival rate between the two groups (p > 0.05). The results of the Cochran-Armitage trend test showed that the 2-year survival rate tended to increase with the duration of Huisheng Oral Liquid treatment (p < 0.05). ③ The Cox proportional -hazards regression model revealed that among all 614 patients, surgery [HR = 0.48, 95% CI = (0.34, 0.68)], chemotherapy [HR = 0.46, 95% CI = (0.31,0.67)], and treatment with Huisheng Oral Liquid for ≥3 months were protective factors [HR = 0.48, 95%CI = (0.27,0.88)], whereas male gender [HR = 1.59, 95% CI = (1.01, 2.50)] and FIB ≥4 g/L [HR = 1.95, 95% CI = (1.37, 2.77)] were risk factors. Conclusion: Chinese patent medicine treatment for ≥3 months showed an improvement in the 2-year survival rate of patients with stage II-III NSCLC. Patients treated with Huisheng Oral liquid for ≥3 months also showed an improvement in the 2-year survival rate, and the 2-year survival rate tended to increase as the treatment duration increased. Finally, male and FIB ≥ 4 g/L were risk factors for prognosis.
Keywords: 2-year survival rate; Chinese patent medicine; Huisheng Oral Liquid; hypercoagulability; non-small cell lung cancer; tumor stage.
Copyright © 2021 Wang, Jia, Li, Zhang, Feng and Du.
Conflict of interest statement
JD, FL, and CZ were employed by Chengdu Diao Pharmaceutical Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Akamatsu H., Mori K., Naito T., Imai H., Ono A., Shukuya T., et al. (2014). Progression-Free Survival at 2 Years Is a Reliable Surrogate Marker for the 5-Year Survival Rate in Patients with Locally Advanced Non-Small Cell Lung Cancer Treated with Chemoradiotherapy. BMC Cancer 14, 18. 10.1186/1471-2407-14-18 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

