Moxibustion improves ovarian function based on the regulation of the androgen balance
- PMID: 34539826
- PMCID: PMC8438671
- DOI: 10.3892/etm.2021.10664
Moxibustion improves ovarian function based on the regulation of the androgen balance
Erratum in
-
Erratum: Moxibustion improves ovarian function based on the regulation of the androgen balance.Exp Ther Med. 2022 Mar;23(3):190. doi: 10.3892/etm.2022.11113. Epub 2022 Jan 4. Exp Ther Med. 2022. PMID: 35126693 Free PMC article.
Abstract
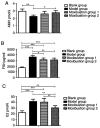
The effect of androgens on follicular development and female reproduction has become an active research topic. Moxibustion is a Traditional Chinese Medicine therapy that has been reported to be able to prevent and treat numerous ovary-related problems. However, studies on the effect of moxibustion for diminished ovarian reserve (DOR) on androgen balance are still lacking. The present study aimed to assess the efficacy of moxibustion intervention prior to disease onset and at the early stage of disease in a rat model of DOR and explore the mechanisms of its effect on ovarian function. A total of 32 rats were randomly divided into four groups: Blank group, Model group (a drug-induced model of DOR), Moxibustion group 1 and Moxibustion group 2. Moxibustion was performed on the BL23 and RN4 acupoints of female rats daily for a total of 20 days (once a day, five times a week for a total of 4 weeks). The two moxibustion groups were established with different intervention times: One group was subjected to pre-disease intervention and the other group to early-disease intervention. The ovarian function was evaluated by detecting anti-Mullerian hormone (AMH), follicle-stimulating hormone (FSH), estradiol (E2), testosterone (T), dehydroepiandrosterone (DHEA), dihydrotestosterone (DHT) and androgen receptor (AR) levels in the serum or the ovary samples. To further investigate the downstream regulatory factors for AR after moxibustion treatment for pre-disease or early-disease intervention, FSH receptor (FSHR) and microRNA (miR)-125b expression in ovaries were also analyzed. The results indicated that AMH and DHT levels were reduced in the model group compared with those in the blank group, while FSH, T and DHEA levels were increased. AMH and DHT levels were increased in Moxibustion group 1 compared with those in the model group, while FSH, T and DHEA levels were reduced. There was no difference in E2 levels between Moxibustion group 1 and the model group. Compared with that in the model group, the AR content in the ovary was increased in Moxibustion group 1. There was no difference in FSHR mRNA in the ovaries between Moxibustion group 1 and the model group. miR-125b levels were significantly increased in Moxibustion group 1 as compared with those in the model group. Furthermore, AMH and DHT levels were increased in Moxibustion group 2 compared with those in the model group, while FSH, T and DHEA levels were reduced. E2 levels were significantly decreased in Moxibustion group 2 compared with those in the model group. The relative mRNA expression of AR, FSHR and miR-125b was decreased following establishment of the model. Compared with that in the model group, the AR content in the ovary was increased in Moxibustion group 2. In comparison with the blank and model groups, the FSHR content in the ovary of Moxibustion group 2 was significantly increased. miR-125b levels were not obviously altered in Moxibustion group 2 as compared with those in the model group. In addition, there was no significant difference in AMH, FSH, T and DHEA levels between the two moxibustion groups. E2 and DHT levels were higher in Moxibustion group 1 than in Moxibustion group 2. There was no difference in AR mRNA expression between the two moxibustion groups. FSHR mRNA levels were lower in Moxibustion group 1 than in Moxibustion group 2, while miR-125b mRNA levels were higher in Moxibustion group 1 than in Moxibustion group 2. In conclusion, the present study suggested that moxibustion intervention prior to disease onset and at the early disease stage was able to improve ovarian function via modulation of the AR-mediated stable equilibrium of androgens. However, the effects and mechanisms of moxibustion intervention for pre-disease and early-disease intervention of DOR appear to be different. The appropriate duration of treatment and the time-effect relationship require to be further studied.
Keywords: androgen balance; diminished ovarian reserve; early disease intervention; moxibustion; pre-disease intervention.
Copyright: © Jin et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Devine K, Mumford SL, Wu M, DeCherney AH, Hill MJ, Propst A. Diminished ovarian reserve in the United States assisted reproductive technology population: Diagnostic trends among 181,536 cycles from the Society for assisted reproductive technology clinic outcomes reporting system. Fertil Steril. 2015;104:612–619.e3. doi: 10.1016/j.fertnstert.2015.05.017. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
