Bioactive Hexapeptide Reduced the Resistance of Ovarian Cancer Cells to DDP by Affecting HSF1/HSP70 Signaling Pathway
- PMID: 34539881
- PMCID: PMC8425193
- DOI: 10.7150/jca.62285
Bioactive Hexapeptide Reduced the Resistance of Ovarian Cancer Cells to DDP by Affecting HSF1/HSP70 Signaling Pathway
Abstract
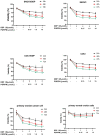
Ovarian cancer is the leading cause of death in gynecologic malignancies. Ovarian cancer as a metastatic malignant tumor is highly recurrent and prone to drug resistance. Bioactive peptides are an emerging area of biomedical research in reducing resistance of tumor cell to drugs. In this paper, we investigated the effects and mechanisms of bioactive hexapeptide (PGPIPN) derived in milk protein on the sensitivity of ovarian cancer cells to cis-dichlorodiammine platinum (DDP). Human ovarian cancer cell lines (SKOV3 and COC1), their DDP-resistant sublines (SKOV3/DDP and COC1/DDP) and human primary ovarian cancer cells were cultured in vitro under the combined treatment of DDP (close to IC50) and different concentrations of PGPIPN. The viabilities, apoptosis and cell cycle changes were respectively measured by WST-8 and flow cytometry. The mRNA and protein expression levels of HSF1, HSP70, MDR1, ERCC1 and β-actin gene were respectively assayed by RT-qPCR and western blotting. The results showed that PGPIPN significantly increased the sensitivity of human ovarian cancer cells to DDP in inhibiting viability and inducing apoptosis in vitro. But the effects in sensitive cells were lower than DDP-resistant cells. PGPIPN significantly changed the cell cycles in all human ovarian cancer cells, which leaded to a significant increase in the percentage of cells blocked at G2/M phase and decrease the percentage of cells at G1 phases in a dose-dependent manner. PGPIPN affected the expression levels of HSF1, HSP70, MDR1 and ERCC1 genes. Compared with cells in DDP treatment alone, the expression levels of HSF1 and HSP70 in human ovarian cancer cells treated with DDP and PGPIPN together significantly decreased in dose-dependent manner. PGPIPN significantly decreased MDR1 and ERCC1 of drug-resistant ovarian cancer cell lines and human primary ovarian cancer cell in a dose-dependent manner. Pifithrin-μ (PFTμ, HSP70 inhibitor) decreased or removed the effects of peptide in increasing the sensitivity of ovarian cancer cells to DDP. This suggests that PGPIPN enhanced the sensitivity of ovarian cancer cells to DDP partially via reducing the activity of HSF1/HSP70 signaling pathway, thus inducing cell apoptosis and decreasing repairment of DNA damage.
Keywords: apoptosis; bioactive hexapeptide; cell cycle; drug resistance; human ovarian cancer; signaling pathway.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Wang W, Cao Y, Zhou X, Wei B, Zhang Y, Liu X. PTP1B promotes the malignancy of ovarian cancer cells in a JNK-dependent mechanism. Biochem Biophys Res Commun. 2018;503:903–9. - PubMed
-
- Dong X, Men X, Zhang W, Lei P. Advances in tumor markers of ovarian cancer for early diagnosis. Indian J Cancer. 2014;51(Suppl 3):e72–6. - PubMed
-
- Guan LY, Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discov Med. 2018;26:219–29. - PubMed
-
- Barnett R. Ovarian cancer. Lancet. 2016;387:1265. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

