Born to Run? Diverse Modes of Epithelial Migration
- PMID: 34540829
- PMCID: PMC8448196
- DOI: 10.3389/fcell.2021.704939
Born to Run? Diverse Modes of Epithelial Migration
Abstract
Bundled with various kinds of adhesion molecules and anchored to the basement membrane, the epithelium has historically been considered as an immotile tissue and, to migrate, it first needs to undergo epithelial-mesenchymal transition (EMT). Since its initial description more than half a century ago, the EMT process has fascinated generations of developmental biologists and, more recently, cancer biologists as it is believed to be essential for not only embryonic development, organ formation, but cancer metastasis. However, recent progress shows that epithelium is much more motile than previously realized. Here, we examine the emerging themes in epithelial collective migration and how this has impacted our understanding of EMT.
Keywords: EMT; apicobasal polarity; cell polarity; collective migration; epithelial polarity; extracellular matrix; mechanosensing; mesenchymal-epithelial transition.
Copyright © 2021 Lu and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
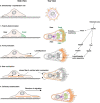
Figures




References
-
- Aoki K., Kondo Y., Naoki H., Hiratsuka T., Itoh R. E., Matsuda M. (2017). Propagating wave of ERK activation orients collective cell migration. Dev. Cell 43 305–317.e5. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

